Figure 4.
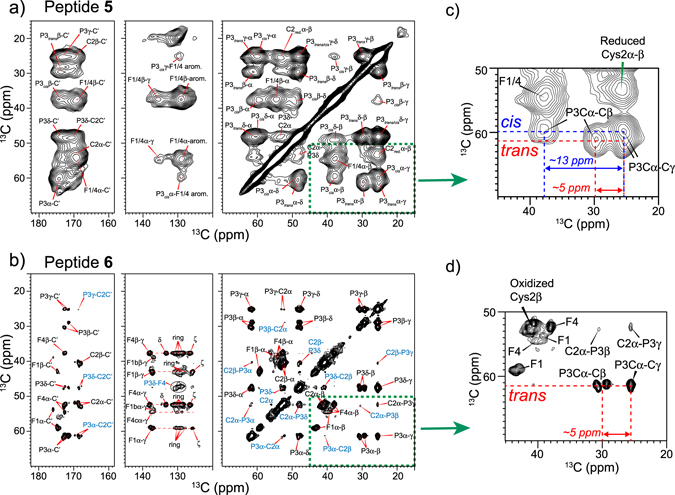
2D 13C-13C correlation spectra of the (a) unreacted π-clamp peptide 5 and (b) PFA-tagged peptide 6. The spectra were acquired at 298 K. (c,d) Enlarged regions showing Pro3 and Cys2 cross peaks. The Cys2 Cβ chemical shift indicates that Cys2 is in a reduced state (~25 ppm) in peptide 5 (c), and in an oxidized state (~40 ppm) in the PFA-tagged peptide 6 (d). The chemical shift difference between Pro Cβ and Cγ indicates the isomer state. The Pro intensity in (c) indicates an about 1:1 ratio between the cis and trans isomers in the unreacted peptide 5. The Pro signal in (d) shows only the trans Pro conformation is adopted in PFA-tagged peptide 6.
