Figure 2.
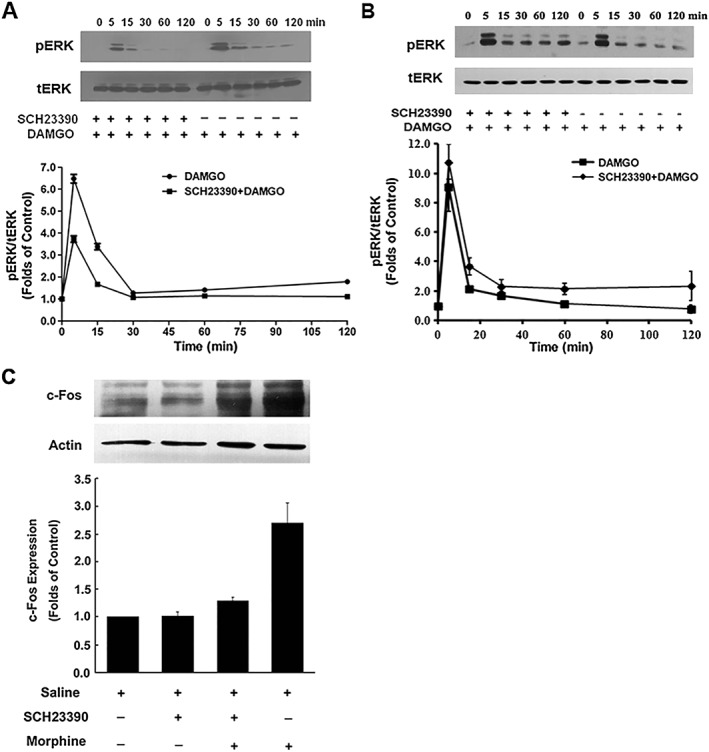
SCH23390 suppressed DAMGO‐mediated activation of ERK1/2 and morphine‐mediated expression of c‐Fos protein. (A, B) SCH23390 attenuated DAMGO‐induced increase in phosphorylated ERK1/2. HEK293 cells expressing μ receptors (μOR) and D1 receptors (D1R) (A) or expressing μ receptors alone (B) were treated with DAMGO (1 μM) for indicated time points in the absence or presence of SCH23390 (1 μM). The phosphorylation of ERK1/2 was determined by immunoblotting and quantified by densitometry. Upper panels show representative blots from Western blotting. Bottom panels show quantification of blots of phospho‐ERK1/2. Values are expressed as the mean ± SEM of three independent experiments. (C) SCH23390 inhibited morphine‐induced increase in c‐Fos protein levels. HEK293 cells expressing μ receptors and D1 receptors were treated with saline or morphine (10 μM) in the presence or absence of SCH23390 (1 μM). After 1 h, nuclear fractionation was isolated. The nuclear fraction (nuclei) and crude extract (total extract) were analysed by SDS‐PAGE and immunoblotted by using rabbit anti‐c‐Fos (1:500) and mouse anti‐actin (1:5000) respectively. The intensities of the immunoreactive bands were quantified by densitometry. Upper panels show representative blots of c‐Fos from Western blotting. Lower panels show quantification of blots of c‐Fos. All values are normalized against actin as a control protein. Values are expressed as the mean ± SEM of three independent experiments.
