Abstract
Background:
Depression in patients with chronic rhinosinusitis (CRS) is underdiagnosed but significantly impacts treatment outcomes and health care utilization.
Objective:
To compare undiagnosed depression in a CRS cohort with a healthy, non-CRS control cohort.
Methods:
A case-control study of patients with symptomatic CRS and a non-CRS control cohort was performed. Demographic and comorbidity factors were correlated to depression-specific outcomes by using the Beck Depression Inventory II (BDI).
Results:
We enrolled 42 patients with CRS and 88 control patients with no history of CRS. Physician-diagnosed depression was equivalent in CRS and control patients (6% and 9%, respectively). BDI-detected depression was higher among patients with CRS compared with controls (31% versus 14.8%, respectively; p = 0.031). BDI scores were higher in patients with CRS even when controlling for comorbid asthma, allergy, and aspirin sensitivity. When examined by polyp status, the patients without polyps had more depression than did the controls (38% versus 14.8%; p = 0.048). The somatic subscale scores of the BDI were worse in patients with CRS (p = 0.004), whereas the cognitive subscale trended toward significance (p = 0.081).
Conclusion:
Depression may be more common in CRS than previously recognized, especially in patients without polyps. Somatic subscale scores of the BDI are increased in CRS and may impact future treatment outcomes.
Keywords: Chronic rhinosinusitis, depression, somatic, affective, polyp
Chronic rhinosinusitis (CRS) is a complex disease that affects not only the nose and sinuses but also has broad effects throughout the body. Previous studies in CRS found that 9–25% of patients report a previous physician diagnosis of depression.1,2 Studies that used validated screening instruments were able to identify an additional 8–15% of patients with CRS with previously undiagnosed depression; however, they did not include a control group of patients without CRS for comparison.1,3,4 When depression occurs in patients with CRS, the depression has been shown to impact quality of life and, potentially, treatment outcomes1,5; thus, a comprehensive understanding of comorbid depression in CRS is needed.
In addition to depression, patients with CRS have other comorbid systemic ailments, including cognitive dysfunction, anxiety, diabetes, and sleep disorders.3,6–8 Although it is unclear how these comorbid systemic conditions, including depression, develop in patients with CRS, it is possible that increased cardinal symptoms, such as nasal obstruction or drainage, could impact sleep, whereas increased facial and/or sinus pain or impaired olfactory function could impact pleasurable social activities. It also is possible that variations in the severity of cardinal symptoms between polyp groups could lead to different mechanisms of depression. Systemic manifestations and comorbidities are likely a major factor in impaired health utility scores in CRS compared with other chronic diseases5 as well as reduced work productivity9 and increased medication usage.5,10
Given the negative impact of comorbid depression on clinical outcomes for CRS, better screening for undiagnosed depression and improved understanding of the complex interplay between CRS-associated depression and accompanying systemic comorbidities are needed. The goals of this study were to examine depression in patients with CRS by using a sensitive instrument, the Beck Depression Inventory II (BDI), to compare depression in patients with CRS with non-CRS controls and to determine if there were variations in depression among the polyp groups.
METHODS
Study Design
The study was designed as a prospective, case-control study of adult patients (≥18 years). Patients with CRS were enrolled from tertiary rhinology clinics at the Medical University of South Carolina, and informed consent was obtained under institutional review board approval (Pro 16334). All patients classified as CRS fulfilled diagnostic criteria for CRS according to the Clinical Practice Guideline of the American Academy of Otolaryngology-Head and Neck Surgery11 and the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS2012),12 with confirmation of disease by either computed tomography or sinonasal endoscopy. Endoscopy was graded by using Lund-Kennedy scoring. Control subjects without any history of sinusitis were recruited from individuals who accompanied patients to otolaryngology appointments at the Medical University of South Carolina, such that both cases and controls were derived from the same theoretical source population. Patients with CRS were excluded if they had received oral corticosteroids within the preceding 2 weeks, had undergone surgery within 6 months, or had a cognitive mental disorder (dementia, delirium, or amnesia). Control subjects were excluded if they had any history of chronic or recurrent sinusitis, ongoing symptoms that fit diagnostic criteria for CRS, or cognitive mental disorder (dementia, delirium, or amnesia).
Demographics, Comorbidities, and Disease Severity
By using standardized questionnaires, demographic information was collected from all the subjects, including age, sex, race and/or ethnicity, educational attainment (International Standard Classification Education), and household income. Information related to medical comorbidities was collected directly from the subjects and the medical records when available, including the presence of allergic rhinitis, asthma, obstructive sleep apnea, aspirin-exacerbated respiratory disease, and diabetes mellitus. Physician-diagnosed depression was based on patient report of depression diagnosis given by their primary care physician or psychiatrist. Allergic rhinitis had to be diagnosed by a physician and confirmed with previous positive objective testing (skin-prick test or allergen-specific immunoglobulin E antibody test). For all other medical comorbidities, the patient was considered to have the condition if he or she had received a previous diagnosis by a physician. We did not include patient self-diagnosis of depression, allergies, or any other comorbidity, and we did not obtain outside medical records to confirm these diagnoses.
Symptoms of depression were evaluated by using the Beck Depression Inventory Second Edition (BDI-II).13 The BDI-II is a 21-item, self-reporting questionnaire that quantifies depressive symptoms over the past 2 weeks. The BDI-II is one of the most widely used instruments for detecting and quantifying the severity of depressive symptoms in normal and depressed populations, and has high internal consistency and reliability. Scores are generally classified as the following: 0–13, minimal or no depression; 14–19, mild depression; 20–28, moderate depression; and 29–63, severe depression.14,15 Subfactor analysis was performed to determine the impact of CRS on somatic and cognitive subdomains.15,16
Statistical Analysis
Data were collected on standardized forms and entered into a Research Electronic Data Capture data base by using double data-entry processes to ensure data integrity.17 Descriptive statistics (means, standard deviations [SD], percentages, etc.) were used to characterize the patients with CRS and the controls with respect to all study measures, including demographics, comorbidities, potential confounding factors, and measures of depression. For continuous measures, normality was assessed by plotting histograms. For each measure, univariate analysis was performed, which compared differences between cases with CRS and the controls. An independent samples t-test was used to compare differences for normally distributed continuous measures, whereas non-normal measures were evaluated by using the Mann-Whitney U test. Frequency data were compared between cases and controls by using the Pearson χ2 test or Fisher's exact test when appropriate. Also, linear regression was performed to determine the extent to which depression measures (BDI and its subscales) were associated with cases.
RESULTS
Overall
We enrolled 42 patients with CRS and 88 controls with no history of CRS. Patients with CRS had a higher prevalence of allergy, asthma, and aspirin-exacerbated respiratory disease (Table 1). Endoscopy was performed in all patients with CRS, and the mean (SD) Lund-Kennedy score was 6.00 ± 3.21. Computed tomography was performed in 30 patients with CRS as part of their routine clinical care, and the mean (SD) Lund-MacKay score was 13.87 ± 6.77 (Table 1). There was no difference between the groups in the prevalence of physician-diagnosed depression, but BDI scores revealed that patients with CRS had depression twice as often as did the control patients (31.0% versus 14.8%, respectively; p = 0.031) (Table 2), which led to a modestly higher mean (SD) BDI score in the CRS cohort (10.62 ± 8.43 versus 6.77 ± 7.52, respectively; p = 0.010). Among patients with BDI scores of ≥14 and thus screened positive for depression, BDI scores were similar between the groups. The mean BDI scores among patients with depression in both groups were >20, which indicated moderate depression (Table 2), which indicated that, although undiagnosed depression was more common in patients with CRS, it was not more severe.14,15
Table 1.
Baseline demographics of healthy control and CRS cohorts
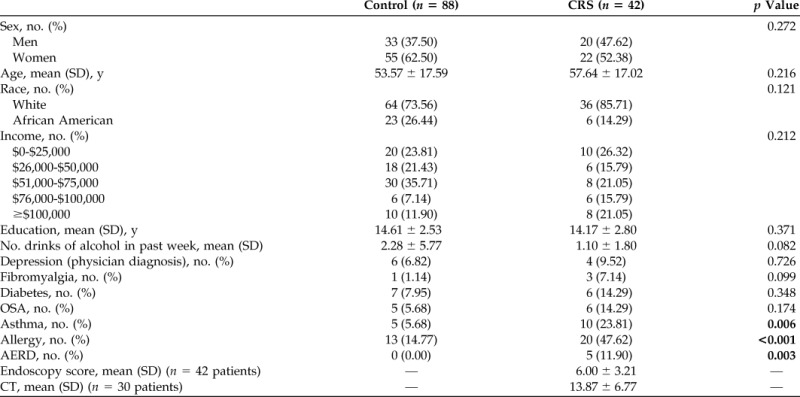
CRS = chronic rhinosinusitis; SD = standard deviation; OSA = obstructive sleep apnea; AERD = aspirin-exacerbated respiratory disease; CT = computed tomography.
Bold indicates statistically significant values.
Table 2.
Depression prevalence and severity by diagnostic group
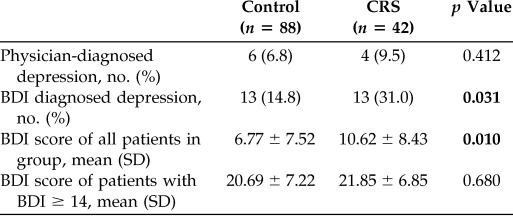
CRS = chronic rhinosinusitis; BDI = Beck Depression Inventory-II; SD = standard deviation.
Bold indicates statistically significant values.
Comorbidities
We then tested for correlation among demographic factors, comorbidities, and BDI scores (Table 3) among all the patients. Physician-diagnosed depression, fibromyalgia, and asthma were associated with higher BDI scores. Physician-diagnosed depression and fibromyalgia did not differ between the groups (Table 1), thus CRS status and asthma were placed into linear regression analysis. CRS remained significantly associated with higher BDI scores even when controlling for asthma (β = 3.289, p = 0.032).
Table 3.
Correlation between BDI scores and demographic variables
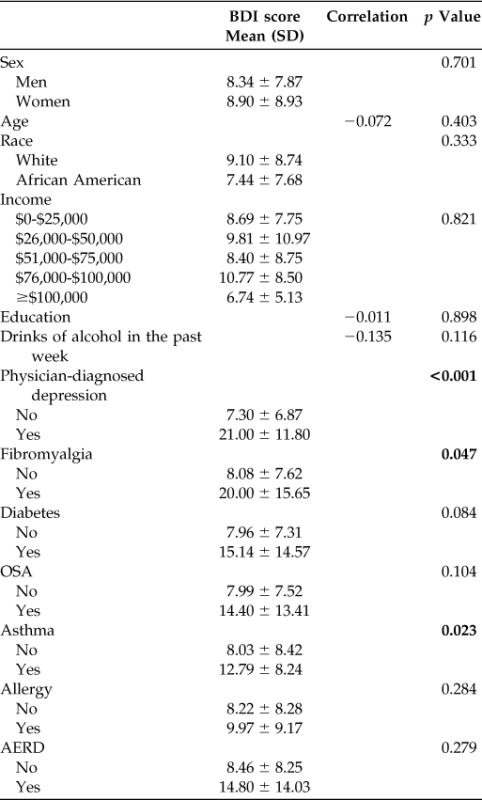
BDI = Beck Depression Inventory-II; SD = standard deviation; OSA = obstructive sleep apnea; AERD = aspirin-exacerbated respiratory disease.
Bold indicates statistically significant values.
BDI Subscales
We then examined whether demographic factors and comorbidities had a differential impact on the somatic or cognitive subscales of the BDI. As seen in Table 4, somatic scores correlated with asthma, physician-diagnosed depression, and CRS diagnosis. Physician-diagnosed depression did not vary between the cohorts, thus asthma and CRS diagnosis were placed into linear regression, and CRS correlated with somatic scores even when controlling for asthma. Cognitive scores trended toward a difference between patients with CRS and the controls but did not reach statistical significance (p = 0.081).
Table 4.
Somatic and cognitive subscale BDI scores among all patients
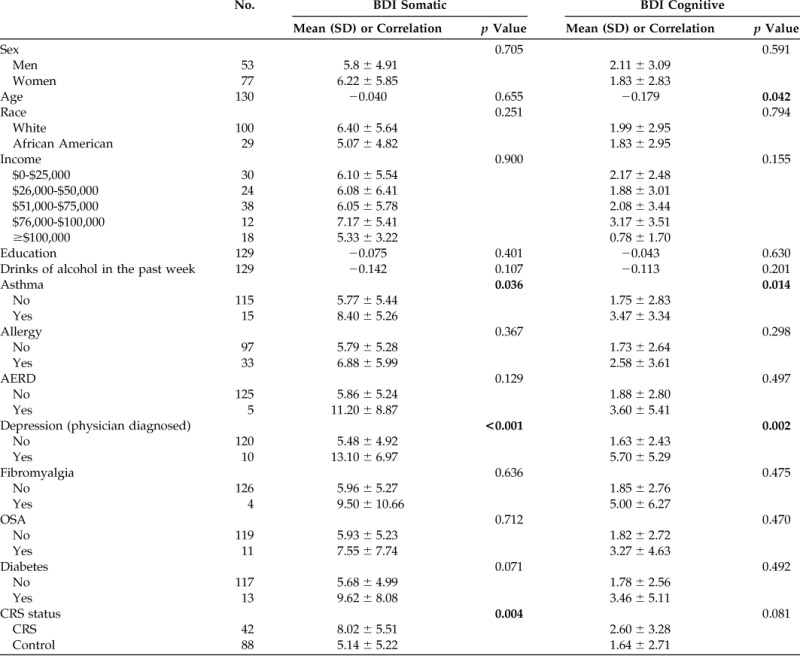
BDI = Beck Depression Inventory-II; SD = standard deviation; AERD = aspirin-exacerbated respiratory disease; OSA = obstructive sleep apnea; CRS = chronic rhinosinusitis.
Bold indicates statistically significant values.
Polyp Status
We then examined polyp status and its impact on BDI scores. As demonstrated in Table 5, although all the groups had equivalent rates of physician-diagnosed depression, CRS without nasal polyps (CRSsNP) had significantly higher rates of BDI diagnosed depression compared with the control patients (38.1% versus 14.8%, respectively; p = 0.048), which resulted in an increase in mean (SD) BDI score compared with the control patients (11.43 ± 7.76 versus 6.77 ± 7.52, respectively; p = 0.029). Patients with CRS with nasal polyps (CRSwNP) had an intermediate prevalence (23.8%) and mean (SD) BDI score (9.81 ± 9.16).
Table 5.
Prevalence of depression and BDI score variations based on polyp status

BDI = Beck Depression Inventory-II; CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; SD = standard deviation.
Bold indicates statistically significant values.
DISCUSSION
A variety of chronic medical conditions are commonly associated with an increased rate of comorbid depression. This includes diabetes, cancer, coronary heart disease, stroke, and rheumatoid arthritis.18–20 It is difficult to determine causality between chronic illness and depression. Patients with depression may have physiologic effects due to their mental illness or maladaptive behaviors, which increase their risk for developing some conditions, such as type 2 diabetes.21 However, depression may actually be an early manifestation or even the initial presentation of an underlying medical condition due to biologic changes or complications seen with these chronic illnesses.22 Comorbid depression has also been shown to impact treatment outcomes in other diseases. Patients with depression have increased death and rehospitalization rates after coronary artery bypass grafting.23 Within this context, it is not surprising that CRS would be associated with a higher prevalence of depression compared with a group of individuals without CRS. Furthermore, it will be valuable to advance our knowledge regarding the prevalence and types of depression in patients with CRS and, ultimately, to determine whether depression is a risk factor for and/or modifies the course of CRS after treatment.
Our study was consistent with previous reports regarding the general prevalence of previous physician-diagnosed depression in patients with CRS, ∼10%,1,2 and this was similar to the prevalence we found in the non-CRS control cohort. Use of the BDI instrument as a screening tool seemed to be more sensitive than other instruments at detecting patients with CRS at risk for depression because our overall prevalence was 31%, one of the highest reported in the literature.1,3,4 It is unclear if this was due to increased sensitivity of the BDI screening instrument or variations in patient populations.
One advantage of our study was inclusion of a non-CRS control group, which enabled us to examine the impact of common CRS-related comorbidities. Asthma, diabetes, and obstructive sleep apnea have all been associated with depression.24–27 Given these findings, we investigated the role of these comorbidities in the development of depression in CRS. We found that, even when controlling for these variables, CRS was still associated with higher BDI scores, which indicated independent effects of CRS on depressive symptoms. One potential weakness in our study was that the control patients were typically accompanying friends or family members and were not true patients in our clinics, thus, we did not access their medical records, which could potentially lead to reporting bias and underreporting of comorbidities.
There are a number of instruments available for screening of depression, which includes physician-administered measures, e.g., the Hamilton Rating Scale for Depression, and patient reported measures, e.g., the 2- or 9-item versions of the Patient Health Questionnaire or the Beck Depression Inventory II (BDI).28 One advantage of the 21-item BDI is that it provides useful information on depression subscales and breaks them down into somatic and cognitive subscales or factors.16 The somatic factor is defined by the core item tiredness or fatigue and also includes questions regarding loss of energy, sleep disturbance, and change in appetite. CRS is known to affect sleep29 and, by definition, includes at least two of the cardinal symptoms of nasal obstruction, drainage, facial pain, and impaired olfaction. Thus, it is not surprising that these sinonasal symptoms could lead to worse somatic scores. The cognitive factor, however, is defined by the core item self-dislike and also includes broader questions, such as pessimism, guilty feelings, and suicidal thoughts. It could be imagined that these symptoms may be more common in many chronic illnesses and may not be as specific for sinus symptoms in CRS.
In our study, the somatic subscale was impacted by CRS. The cognitive subscale was not significantly associated with CRS status, although there was a trend (p = 0.081). Differentiation between these subscales may be important in understanding the development of depression and subsequent treatment outcomes. In other diseases, e.g., development of insulin resistance, patients with worse somatic symptoms rather than cognitive symptoms were more likely to develop diabetes.30 In obstructive sleep apnea, surgical therapy improved BDI scores in 75% of patients with depression and correlated with sleepiness scores. Thus, an impact on somatic symptoms in obstructive sleep apnea seems to translate into improved overall depression symptoms.27 In CRS, the somatic and cognitive subscales may also differentially impact mechanisms for the development of depression. Similarly, whether CRS-related treatments have a greater impact on one BDI subscale or another and subsequent depression outcomes is an interesting area for future research.
Previous uncontrolled studies that used other depression instruments did not find any impact from nasal polyp status. In contrast, we found that CRSsNP had an increased prevalence of depression compared with healthy controls. Patients with CRSwNP tend to present with more severe symptoms of nasal congestion and olfactory loss, whereas patients with CRSsNP can have more severe pain.31 It is unclear if certain cardinal symptoms play a more pivotal role in the development of depression. Similarly, variability in the severity of cardinal symptoms between the polyp groups could result in variability in somatic symptoms and overall BDI scores. Also, other comorbidities, such as asthma and allergies, are more often associated with patients with CRSwNP and could certainly contribute to overall depression. We were able to control for a number of these comorbidities, but it is possible that there were other comorbidities not included in our study.
Medical and surgical therapies impact cardinal symptoms of CRS with varying degrees of success,32,33 e.g., impaired olfaction tends to improve less than other cardinal symptoms.32 Thus, a more comprehensive understanding of which BDI subscales play key roles in CRS and which subscales impact depression outcomes is needed. We did not analyze individual cardinal symptoms, but this remains an area for further investigation. It would also be interesting to determine if other otolaryngologic symptoms, such as ear symptoms or nasal obstruction due to septal deviation or rhinitis, are associated with depression.
Although our study was unique in comparing depression in patients with CRS with non-CRS controls when using the comprehensive BDI, it did have a number of weaknesses. First, the study was not powered to control for a large number of variables, such as polyp status, sinus-specific symptoms, and depression subscales. Second, for physician-diagnosed depression, we relied on the patient report of a previous physician diagnosis. We did not collect detailed data regarding the criteria used by other physicians in rendering this diagnosis or therapies recommended for treatment. Third, a large number of chronic illnesses have been associated with depression. Our study was limited to those illnesses commonly associated with CRS. It is possible that other comorbidities, such as cardiac disease or low back pain, could also be present but were not accounted for. Similarly, it is possible that multiple combinations of these comorbidities, such as asthma and cardiac disease, could impact depression outcomes. As we begin to better understand depression in CRS, it will be important to have larger, multi-institutional studies. Such detailed studies will enable us to understand the development of CRS-associated depression and will also be critical to determine the impact of medical and surgical treatments for CRS on depression outcomes.
CONCLUSION
Use of the BDI as a screening instrument detected depression in nearly one-third of the patients with CRS. Depression in these patients was independent of other chronic illnesses associated with CRS, including asthma and allergic rhinitis, and seemed to impact the somatic subscale of the BDI. Depression seemed to be more common in patients with CRSsNP.
Footnotes
Supported by a grant from the Flight Attendant Medical Research Institute (ID113042_CIA). Z.M. Soler is also supported by grants from the National Institute on Deafness and Other Communication Disorders, one of the National Institutes of Health, Bethesda, Maryland (R01 DC005805; R03 DC013651–01). B.M. Cortese is supported by a grant from the National Institute of Mental Health (K01 MH090548).
Z.M. Soler is a consultant for Olympus, which is not affiliated with this manuscript, and has grant support from Entellus and IntersectENT. R.J. Schlosser is supported by grants from OptiNose, Entellus, and IntersectENT, which are not associated with this manuscript, and is a consultant for Olympus, Meda, and Arrinex, which are not affiliated with this study. The remaining authors have no conflicts of interest pertaining to this article
REFERENCES
- 1. Litvack JR, Mace J, Smith TL. Role of depression in outcomes of endoscopic sinus surgery. Otolaryngol Head Neck Surg 144:446–451, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mace J, Michael YL, Carlson NE, et al. Effects of depression on quality of life improvement after endoscopic sinus surgery. Laryngoscope 118:528–434, 2008. [DOI] [PubMed] [Google Scholar]
- 3. Tomoum MO, Klattcromwell C, DelSignore A, et al. Depression and anxiety in chronic rhinosinusitis. Int Forum Allergy Rhinol 5:674–681, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Nanayakkara JP, Igwe C, Roberts D, Hopkins C. The impact of mental health on chronic rhinosinusitis symptom scores. Eur Arch Otorhinolaryngol 270:1361–1364, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Brandsted R, Sindwani R. Impact of depression on disease-specific symptoms and quality of life in patients with chronic rhinosinusitis. Am J Rhinol 21:50–54, 2007. [DOI] [PubMed] [Google Scholar]
- 6. Hajjij A, Mace JC, Soler ZM, et al. The impact of diabetes mellitus on outcomes of endoscopic sinus surgery: A nested case-control study. Int Forum Allergy Rhinol 5:533–540, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soler ZM, Wittenberg E, Schlosser RJ, et al. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope 121:2672–2678, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soler ZM, Eckert MA, Storck K, Schlosser RJ. Cognitive function in chronic rhinosinusitis: A controlled clinical study. Int Forum Allergy Rhinol 5:1010–1017, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Rudmik L, Smith TL, Schlosser RJ, et al. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope 124:2007–2012, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wasan A, Fernandez E, Jamison RN, Bhattacharyya N. Association of anxiety and depression with reported disease severity in patients undergoing evaluation for chronic rhinosinusitis. Ann Otol Rhinol Laryngol 116:491–497, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Rosenfeld RM. Clinical practice guideline on adult sinusitis. Otolaryngol Head Neck Surg 137:365–377, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 50:1–12, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Bringmann LF, Lemmens LH, Huibers MJ, et al. Revealing the dynamic network structure of the Beck Depression Inventory-II. Psychol Med 45:747–757, 2015. [DOI] [PubMed] [Google Scholar]
- 14. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67:588–597, 1996. [DOI] [PubMed] [Google Scholar]
- 15. Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Rev Bras Psiquiatr 35:416–431, 2013. [DOI] [PubMed] [Google Scholar]
- 16. Huang C, Chen JH. Meta-analysis of the factor structures of the Beck Depression Inventory-II. Assessment 22:459–472, 2015. [DOI] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Zwaan GL, van Dijk SE, Adriaanse MC, et al. Diagnostic accuracy of the Patient Health Questionnaire-9 for assessment of depression in type II diabetes mellitus and/or coronary heart disease in primary care. J Affect Dis 190:68–74, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Malhotra R, Chei CL, Menon E, et al. Short-term trajectories of depressive symptoms in stroke survivors and their family caregivers. J Stroke Cerebrovasc Dis 25:172–181, 2016. [DOI] [PubMed] [Google Scholar]
- 20. Iaquinta M, McCrone S. An integrative review of correlates and predictors of depression in patients with rheumatoid arthritis. Arch Psychiatr Nurs 29:265–278, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Berge LI, Riise T. Comorbidity between Type 2 Diabetes and Depression in the Adult Population: Directions of the Association and Its Possible Pathophysiological Mechanisms. Int J Eendocrinol 2015:164760, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci 13:7–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stenman M, Holzmann MJ, Sartipy U. Relation of major depression to survival after coronary artery bypass grafting. Am J Cardiol 114:698–703, 2014. [DOI] [PubMed] [Google Scholar]
- 24. Boudreau M, Bacon SL, Ouellet K, et al. Mediator effect of depressive symptoms on the association between BMI and asthma control in adults. Chest 146:348–354, 2014. [DOI] [PubMed] [Google Scholar]
- 25. Katotomichelakis M, Simopoulos E, Zhang N, et al. Olfactory dysfunction and asthma as risk factors for poor quality of life in upper airway diseases. Am J Rhinol Allergy 27:293–298, 2013. [DOI] [PubMed] [Google Scholar]
- 26. Nicolau J, Rivera R, Frances C, et al. Treatment of depression in type 2 diabetic patients: Effects on depressive symptoms, quality of life and metabolic control. Diabetes Res Clin Pract 101:148–152, 2013. [DOI] [PubMed] [Google Scholar]
- 27. Ishman SL, Benke JR, Cohen AP, et al. Does surgery for obstructive sleep apnea improve depression and sleepiness? Laryngoscope 124:2829–2836, 2014. [DOI] [PubMed] [Google Scholar]
- 28. Furukawa TA. Assessment of mood: Guides for clinicians. J Psychosom Res 68:581–589, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope 123:2364–2370, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khambaty T, Stewart JC, Muldoon MF, Kamarck TW. Depressive symptom clusters as predictors of 6-year increases in insulin resistance: Data from the Pittsburgh Healthy Heart Project. Psychosom Med 76:363–369, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soler ZM, Smith TL. Quality of life outcomes after functional endoscopic sinus surgery. Otolaryngol Clin North Am 43:605–612, x, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeConde AS, Mace JC, Alt JA, et al. Investigation of change in cardinal symptoms of chronic rhinosinusitis after surgical or ongoing medical management. Int Forum Allergy Rhinol 5:36–45, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soler ZM, Mace J, Smith TL. Symptom-based presentation of chronic rhinosinusitis and symptom-specific outcomes after endoscopic sinus surgery. Am J Rhinol 22:297–301, 2008. [DOI] [PubMed] [Google Scholar]


