Abstract
Bacteria that produce the broad-spectrum Carbapenem antibiotic New Delhi Metallo-β-lactamase (NDM) place a burden on health care systems worldwide, due to the limited treatment options for infections caused by them and the rapid global spread of this antibiotic resistance mechanism. Although it is believed that the associated resistance gene blaNDM-1 originated in Acinetobacter spp., the role of Enterobacteriaceae in its dissemination remains unclear. In this study, we used whole genome sequencing to investigate the dissemination dynamics of blaNDM-1-positive plasmids in a set of 21 clinical NDM-1-positive isolates from Colombia and Mexico (Providencia rettgeri, Klebsiella pneumoniae, and Acinetobacter baumannii) as well as six representative NDM-1-positive Escherichia coli transconjugants. Additionally, the plasmids from three representative P. rettgeri isolates were sequenced by PacBio sequencing and finished. Our results demonstrate the presence of previously reported plasmids from K. pneumoniae and A. baumannii in different genetic backgrounds and geographically distant locations in Colombia. Three new previously unclassified plasmids were also identified in P. rettgeri from Colombia and Mexico, plus an interesting genetic link between NDM-1-positive P. rettgeri from distant geographic locations (Canada, Mexico, Colombia, and Israel) without any reported epidemiological links was discovered. Finally, we detected a relationship between plasmids present in P. rettgeri and plasmids from A. baumannii and K. pneumoniae. Overall, our findings suggest a Russian doll model for the dissemination of blaNDM-1 in Latin America, with P. rettgeri playing a central role in this process, and reveal new insights into the evolution and dissemination of plasmids carrying such antibiotic resistance genes.
Keywords: metallo-beta-lactamase, genomics, antibiotic resistance, Providencia rettgeri, mobile genetic elements, bacterial evolution
Introduction
In the first global report on antimicrobial resistance issued by the World Health Organization very high resistance rates were found in the bacteria that are the main causes of community and health care associated infections, including Enterobacteriacea and Acinetobacter baumannii (World Health Organization 2014). For instance, the prevalence of Enterobacteriaceae resistant to broad-spectrum β-lactam type antibiotics often used as “last-line-of-defence” agents, such as carbapenems, are persistently increasing across the world (Rhomberg and Jones 2009; Prabaker and Weinstein 2011; van Duijn et al. 2011; CDC 2013). Infections caused by carbapenem-resistant bacteria increase health care costs by requiring hospitalization of patients and increase the risk of mortality (Lemos et al. 2014; World Health Organization 2014). It is therefore important to gain greater insight into how resistance spreads, recognizing in doing so that resistance can spread either vertically, through distribution of clones of established “successful” resistant bacterial species, or horizontally, through dispersal of mobile genetic elements (e.g., transposons, plasmids, and prophages) carrying genes for antimicrobial resistance (Woodford et al. 2011). The horizontal transfer of antibiotic resistance genes between bacteria can contribute rapid expansion in the suite of resistance mechanisms present in a bacterial strain.
One mechanism for resistance that can be acquired from the mobile gene pool is the capability for drug modification, an example of which is the group of metallo-β-lactamases. These enzymes have great impact on public health due to their broad substrate range, and are increasing in frequency in clinically important Gram-negative bacteria (Palzkill 2013). A new member of this group of enzymes was identified in 2008 in a patient treated in New Delhi, India (Yong et al. 2009), and named New Delhi Metallo-β-lactamase (NDM). Initially, NDM dissemination was epidemiologically linked to the Indian subcontinent, though the complexity of transmission of this antibiotic resistance determinant became apparent rapidly, due to the presence of NDM-encoding genes (blaNDM) in diverse Gram-negative bacteria, both fermenters (Enterobacteriaceae) and nonfermenters (Acinetobacter baumannii and Pseudomonas aeruginosa) (Johnson and Woodford 2013). Subsequently, NDM-positive strains were isolated in multiple countries on all continents in a great variety of bacterial genera without any epidemiological or molecular links to the strains circulating in the Indian sub-continent (Johnson and Woodford 2013).
The blaNDM gene is usually carried by conjugative plasmids, although the host plasmid characteristics can vary greatly in attributes such as size, incompatibility group, gene content, and organization. As conjugative plasmids are self-transmitting, this underscores the point that these resistance genes can spread independently of clones of the original bacterial host. In Acinetobacter spp., although the blaNDM gene has been reported to be located in the chromosome (Espinal et al. 2011), it mainly resides in a family of plasmids known as pNDM-BJ01-like, named after the first completely sequenced Acinetobacter spp. NDM-plasmid, reported in 2012 (Hu et al. 2012). The pNDM-BJ01-like plasmids are highly conserved, with >99% nucleotide identity extending over at least 85% of the 47-kb pNDM-BJ01 length; they do not belong to any reported incompatibility (Inc) group (Hu et al. 2012); they have a Type IV Secretion System (T4SS); and they have a region of replication and transfer genes separated by a variable region containing a Tn125 composite transposon (Hu et al. 2012). It is within Tn125 that blaNDM is located, along with other genes conserved in the order 5′-blaNDM-bleMBL-trpF-tat-dct-groES-groEL-ISCR21-Δpac-3′, flanked upstream and downstream by ISAba125, though the Tn125 structure has been found to be truncated in some strains. Among non-Acinetobacter bacteria—with the exception of Enterobacter aerogenes (Chen et al. 2015) where blaNDM-1 was found in a pNDM-BJ01-like plasmid—blaNDM is carried in a great variety of plasmids belonging to diverse Inc groups (FII, FIB, A/C2, HI1A, HI1B, L/M, N, N2, X3, R, T as well as unclassified plasmids) (Johnson and Woodford 2013; Khong et al. 2016). Despite the diversity of NDM-encoding plasmids in non-Acinetobacter, the immediate genetic context of the blaNDM gene remains the same in all known cases to date, in that it is always found within Tn125 or its remnants (Poirel et al. 2011; Partridge and Iredell 2012; Wailan, Paterson, et al. 2016). However, Tn125 is often surrounded by other transposons (Tn) or insertion sequence (IS) elements, including ISKpn14, IS26, IS5, ISCR1 or Tn3-like elements, which are frequently found in Enterobacteriaceae and may be involved in the further dissemination of blaNDM through a combination of transposition and homologous recombination (Toleman et al. 2012; Khong et al. 2016; Wailan, Sidjabat, et al. 2016). Although the blaNDM gene is believed to have originated in an Acinetobacter spp. as the result of the fusion of an aminoglycoside resistance gene with a pre-existing metallo-β-lactamase (Toleman et al. 2012) and later transferred to Enterobacteriaceae, aside from the Tn125 remnants and the case of the Enterobacter aerogenes harboring a pNDM-BJ01-like plasmid, little is known about this transmission from Acinetobacter spp. to Enterobacteriaceae, nor about how the diverse blaNDM positive plasmids in Enterobacteriaceae evolved.
NDM-positive Enterobacteriaceae, particularly Providencia spp. play an increasingly important role in multidrug resistant infections and dissemination of blaNDM around the world, as evidenced by the rapidly accumulating reports of isolation of this bacteria harboring this gene (Carvalho-Assef et al. 2013; Mataseje et al. 2014; Pollett et al. 2014; Tada et al. 2014; Carmo Junior et al. 2015; Manageiro et al. 2015; Nachimuthu et al. 2015; Wailan, Paterson, et al. 2016). Previously, we reported the first South American blaNDM-1 outbreak, which occurred in Colombia in Klebsiella pneumonia, as well as an NDM-1-positive Providencia rettgeri outbreak in Mexico, both of which occurred in 2011–2012, without any link to the Indian subcontinent (Barrios et al. 2013; Escobar Perez et al. 2013). Shortly thereafter, we commenced a surveillance study across Colombia, and found the majority of NDM-positive bacteria isolated were P. rettgeri. Here we describe the use of whole genome sequencing (WGS) to investigate the dissemination dynamics of blaNDM-1-positive plasmids among Enterobacteriaceae and A. baumannii clinical isolates from this surveillance study, as well as from the previous outbreaks in Mexico and Colombia. Our results demonstrate interesting genetic links between NDM-1-positive P. rettgeri from distant geographic locations, and between their plasmids and those present in K. pneumoniae and Acinetobacter spp. isolates, providing insights into the central role of P. rettgeri in antibiotic resistance dissemination in Latin America.
Materials and Methods
Isolate Collection and Culture Conditions
Twenty-one NDM-1-positive clinical isolates were included in this study (supplementary data set 1, Supplementary Material online, strains used in this study and statistics of assemblies): P. rettgeri (14), K. pneumoniae (6), and A. baumannii (1). Of these, 12 (11 P. rettgeri and one A. baumannii) were isolated from samples obtained in a surveillance study for carbapenem resistant bacteria that was conducted over a period of 20 months, from September 2012 to April 2014, in three different hospitals in three distant cities in Colombia (Bogota, Cali, and Bucaramanga) (supplementary data set 1, Supplementary Material online). The other nine clinical isolates were obtained from two, previously described clinical outbreaks generated by blaNDM-1 positive K. pneumoniae and P. rettgeri, respectively, reported in Colombia and Mexico (Barrios et al. 2013; Escobar Perez et al. 2013). Clinical and epidemiological features of the all blaNDM-1 positive isolates are listed in supplementary data set 1, Supplementary Material online. Escherichia coli transconjugants were obtained from six representative samples using as donor the blaNDM-1 positive clinical isolate and as recipient the sodium azide-resistant E. coli J53 strain. Equal amounts of a four hours Luria–Bertani (LB) (Oxoid Limited) broth culture of both donor and recipient, were mixed and 100 µl were placed onto a LB agar plate, then conjugation was allowed for 16 h at 37 °C. Subsequently, the NDM-1-positive sodium azide-resistant E. coli transconjugants were selected using LB agar plates supplemented with ceftazidime (30 μg/ml) and sodium azide (100 μg/ml) (Sigma–Aldrich Co. LLC.). The E. coli species (uidA gene) and the blaNDM-1 gene were verified in the transconjugants by PCR. Possible donor strain contamination in the transconjugants was ruled out by PCR using specific primers to the genes khe for K. pneumoniae, gyrB for A. baumannii, and dnaA for P. rettgeri (see supplementary table S1, Supplementary Material online). Otherwise indicated, all NDM-1-positive bacteria were routinely grown in brain hearth infusion agar or broth supplemented with ceftazidime (30 μg/ml) as a selective pressure for guarantee of plasmid permanence.
Whole Genome Sequencing
Total DNA was extracted from 21 blaNDM-1 positive clinical isolates and six transconjugants (supplementary data set 1, Supplementary Material online) using the PureLink® Genomic DNA mini kit from ThermoFisher. Multiplexed total DNA libraries were prepared using the Nextera XT Library Preparation Kit and 300-bp paired end sequencing was performed on the Illumina MiSeq platform using the MiSeq v3 600-cycle reagent kit. Sequencing reads were trimmed and filtered using cutadapt v1.1.7 (Martin 2011) to remove adapters, and PRINSEQ-lite v0.20.4 (Schmieder and Edwards 2011) to remove any low quality reads with average read quality less than Q20, low quality trailing ends with base quality less than Q20 and short reads <87 bp. Reads were then de novo assembled using SPAdes v3.5.0 (Bankevich et al. 2012) with default settings and the assemblies were improved to high-quality draft genome standard (Chain et al. 2009) by scaffolding using SSPACE v2.0 (Boetzer et al. 2011), gap filling using GapFiller v1.10 (Boetzer and Pirovano 2012) and removal of contigs shorter than 300 bp. Details of the sequencing data, assemblies and accession numbers for each of these genomes are listed in supplementary data set 1, Supplementary Material online. The blaNDM-1 gene variant was verified by comparing the genome assemblies against the reported sequence (accession NC_015872) using BLASTn (Altschul et al. 1990). Assembly for each strain was searched for matches to any known blaNDM-positive plasmids, by BLASTn (Altschul et al. 1990) against an extensive database compiled from all fully sequenced blaNDM-carrying plasmids deposited in the NCBI nucleotide repository (a total of 141 complete plasmids as at 18 May 2017; supplementary data set 2, Supplementary Material online).
Phylogenetic Analysis of Providencia rettgeri Clinical Isolates
Since a MLST scheme for the phylogenetic characterization of P. rettgeri isolates does not exist, was built a phylogenetic tree based on the core-genome SNPs determined from the assembled contigs of the 14 P. rettgeri genomes sequenced in this study, plus the draft genome of P. rettgeri Dmel1 (NZ_AJSB00000000.1), the most complete published P. rettgeri genome available at the time, as an out-group control. To build the phylogenetic tree, partially assembled genomes were annotated using Prokka v1.11 (Seemann 2014) and an alignment of concatenated core genes (genes present in all genomes with ≥90% of nucleotide identity) was created with Roary (Page et al. 2015) using PRANK (Loytynoja 2014). Poorly aligned positions and divergent regions were eliminated using Gblocks (Talavera and Castresana 2007). Finally, the phylogenetic tree was created using RAxML version 8.2.9 (Stamatakis 2014) running 1,000 bootstrap replicates under the generalized time reversible model (GTRCAT). Finally, the consensus tree was plotted using Dendroscope (Huson and Scornavacca 2012). Branch lengths are expressed in units of changes/nucleotide position (scale bar).
Complete Plasmid Sequencing
Total DNA was extracted from three representative P. rettgeri isolates (16Pre36, RB151, and 06-1619) using the UltraClean® Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc.). BluePippin (Sage Science) 20-kb size-selected libraries were prepared, then sequenced using one SMRT cell each on the PacBio RS II platform (Pacific Biosciences) using P6-C4 chemistry. Sequencing reads were processed and de novo assembled using the HGAP 3 program of SMRT Analysis v2.3 (Chin et al. 2013) with default parameters. To check the assemblies, the filtered PacBio subreads were mapped to the genome assemblies using BWA-MEM (http://bio-bwa.sourceforge.net/bwa.shtml). The assembly was visually inspected and manually verified using Tablet v1.15.09.01 (Milne et al. 2013). Misassembled terminal repeat overlap sequences, known to be an error of the HGAP assembly of circular molecules (Chin et al. 2013; Hunt et al. 2015), were identified and subsequently trimmed manually. Circularization results were verified using Circlator (Hunt et al. 2015), confirming that the manual assembly correction correlated with the automated method. The complete sequences of five plasmids were confirmed: two plasmids for the strain 16Pre36, one plasmid for strain RB151 and two plasmids for strain 06-1619 (table 1). As the sequence start point of assemblies are arbitrary, the position one of each plasmid was shifted according to the repA gene (pRB151-NDM and p16Pre36-2), pPrY2001 (p16Pre36-NDM and p06-1619-2) or pNDM-BJ01 (p06-1619-NDM) to facilitate comparative genomics. The plasmids were annotated using Prokka v1.11 (Seemann 2014) and manual curation of the automated annotation was facilitated using Artemis (Rutherford et al. 2000). Antibiotic resistance genes were identified using ARIBA (https://github.com/sanger-pathogens/ariba/wiki) and insertion sequence (IS) elements and transposons (Tn) were identified using ISfinder (Siguier et al. 2012) and BLASTn (Altschul et al. 1990). Presence of class 1, 2 or 3 integrons was determined in silico using the primers reported by Marquez et al. (2008).
Table 1.
General features of blaNDM-1-positive plasmids harboured in the strains included in this study.
| Plasmid | Size (bp) | Inc Group | Host | Resistance Gene Profile | GenBank Accesion No | Reference |
|---|---|---|---|---|---|---|
| pNDM-BJ01 | 47,274 | Not assigned | Acinetobacter spp. | aph(3′)-VIa, blaNDM-1 | NC_019268 | Hu et al. (2012) |
| p6234-178kb | 178,193 | IncA/C2 | K. pneumoniae | aph(3′)-VIa, aacA29, aadA2, blaNDM-1, blaCARB-2, mph(E), msr(E), catB3, cmlA1, sul2, sul1 | NZ_CP010391 | Rojas et al. (2016) |
| p16Pre36-NDM | 244,116 | Not assigned | P. rettgeri | aadA1, aph(3′)-Ia, blaNDM-1, sul2, sul1, tet(B), dfrA1 | KX832927 | This study |
| p16Pre36-2 | 43,191 | Not assigned | P. rettgeri | aac(3)-IIa, blaTEM-1B | KX832926 | This study |
| pRB151-NDM | 108,417 | Not assigned | P. rettgeri | blaNDM-1 | CP017672 | Marquez-Ortiz et al. (2017) |
| p06-1619-NDM | 54,712 | Not assigned | P. rettgeri | aph(3′)-VIa, blaNDM-1 | KX832928 | This study |
| p06-1619-2 | 90,666 | Not assigned | P. rettgeri | No resistance genes | KX832929 | This study |
| pPrY2001 | 113,295 | Not assigned | P. rettgeri | aph(3′)-VIa, armA, aacA4, blaNDM-1, aac(6′)Ib-cr, mph(E), msr(E), sul1 | NC_022589 | Mataseje et al. (2014) |
Plasmids sequenced in this study are shown in bold letters. The blaNDM-1-positive pPrY2001 plasmid reported previously in a P. rettgeri from Canada was also included.
Comparative Genomics
We used mapping of consensus data from the MiSeq libraries to explore our set of samples for the presence (or residues) of Colombian and Mexican blaNDM-1-positive sequenced plasmids (table 1) and other related blaNDM-1-positive (pPrY2001 and pNDM-BJ01) and blaNDM-1-negative (p06-1619-2) plasmids. For use in the mapping consensus, a reference database was generated using the concatenated complete sequence of the plasmids p6234-178kb, p16Pre36-NDM, pRB151-NDM, p06-1619-NDM, p06-1619-2, pPrY2001 and pNDM-BJ01, broken in fragments of 300 bp (x axis). This reference database was mapped with SHRiMP2 (David et al. 2011) and Nesoni (https://github.com/Victorian-Bioinformatics-Consortium/nesoni) against the total MiSeq reads from each sample (y axis). The presence of ≥ 90% nucleotide identity when comparing each 300-bp window from the reference plasmids against the consensus generated from MiSeq reads was determined and visualized as black blocks using SeqFindR (http://github.com/mscook/seqfindr). It was included as internal control MiSeq simulated reads to the reference plasmids, generated with ART read simulator (Huang et al. 2012). Pairwise plasmid comparisons, verification of SeqFindR results and figures were performed by using BLASTn (Altschul et al. 1990), ACT (Carver et al. 2005), Easyfig (Sullivan et al. 2011), and BRIG (Alikhan et al. 2011).
Results
The blaNDM-1 Gene Is in a Conjugative Element in the Colombian and Mexican Isolates
From a surveillance study in Colombia a total of 12 NDM-positive isolates were collected from 12 patients (one NDM-positive strain per patient). Among these 12 isolates, which were mostly from outpatients with wound or urinary tract infections, 11 were P. rettgeri (five from Bucaramanga and six from Bogota) and one was A. baumannii isolated in Cali (supplementary data set 1, Supplementary Material online). Additionally, six K. pneumoniae and three P. rettgeri from previously reported (Barrios et al. 2013; Escobar Perez et al. 2013) clinical outbreaks in a neonatal unit in Bogota (Colombia) and an intensive care unit in Monterrey (Mexico), respectively, were also included in this study. The K. pneumoniae and P. rettgeri from Bogota were isolated in the same hospital (supplementary data set 1, Supplementary Material online). Analyses of PCR products confirmed the blaNDM-1 variant was present in all isolates. Thus, in total, 21 NDM-1-positive isolates from Colombia and Mexico were available to investigate in this study.
To explore if the blaNDM-1 gene in the Latin American isolates could be transferred between bacteria, conjugation experiments were performed using six representative NDM-1-positive isolates as donor strains: one representing the K. pneumoniae from Bogota, one the A. baumannii from Cali, one the P. rettgeri from Bogota, two representing the P. rettgeri from Bucaramanga and one the P. rettgeri from Mexico, with the sodium azide-resistant Escherichia coli strain J53 used as the recipient strain. Escherichia coli NDM-1-positive transconjugants were obtained from all six of the donor strains (supplementary data set 1, Supplementary Material online). These results indicate that the blaNDM-1 gene is located in a conjugative element in the Colombian and Mexican isolates, and that it can be transferred to other strains allowing dissemination within and between genera. To determine the relationship among the conjugative blaNDM-1-positive genetic structures and among the NDM-1-positive strains circulating in Latin America WGS was performed on the set of 21 Latin American NDM-1-positive clinical isolates as well as the six NDM-1-positive E. coli transconjugants (supplementary data set 1, Supplementary Material online).
Acinetobacter baumannii 19Aba78 Harbors blaNDM-1 within a pNDM-BJ01-Like Plasmid
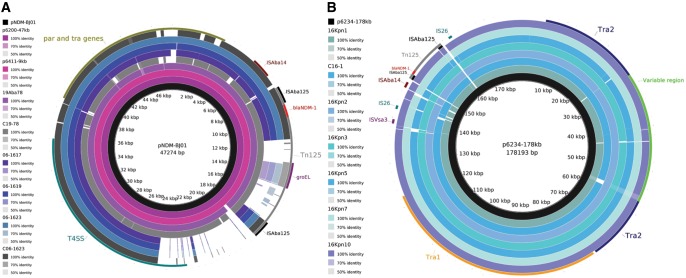
The WGS assembly of A. baumannii isolate 19Aba78 from Cali, Colombia, had 100% nucleotide identity over 99% of the pNDM-BJ01 length (fig. 1A and supplementary data set 2, Supplementary Material online), suggesting that blaNDM-1 is located on a pNDM-BJ01-like plasmid, as has been broadly reported in Acinetobacter spp. (Hu et al. 2012; Sun et al. 2013; Espinal et al. 2015). The 19Aba78 pNDM-BJ01-like plasmid assembled into seven contigs, so the location of all these contigs together on a single plasmid cannot be confirmed from the current assembly. Of note however, blaNDM-1 is located within Tn125 adjacent to plasmid sequences that are identical to pNDM-BJ01, on a single contig in the 19Aba78 assembly (see supplementary fig. S1A, Supplementary Material online), supporting the presence of blaNDM-1 on a pNDM-BJ01-like plasmid. Additionally, the E. coli transconjugant C19-78 allowed confirmation of blaNDM-1 located on a pNDM-BJ01-like plasmid (fig. 1A and supplementary data set 2, Supplementary Material online). All contigs that mapped to the genome sequence of the recipient strain, E. coli J53 (accession AICK00000000), were removed from the WGS assembly of the transconjugant C19-78, and all remaining contigs were found to map to pNDM-BJ01, confirming the pNDM-BJ01-like plasmid harbored by C19-78 had no insertions or additional sequences (see supplementary fig. S1A, Supplementary Material online).
Fig. 1.
—blaNDM-1-plasmids circulating among Acinetobacter baumannii and Klebsiella pneumoniae in Colombia. (A) BLASTn comparison of WGS assemblies of A. baumanni 19Aba78 from this study and its Escherichia coli transconjugant C19-78, the Providencia rettgeri NDM-positive isolates from Mexico (06-1617, 06-1619 and 06-1623) and the E. coli transconjugant C06-1623 against the plasmid pNDM-BJ01. Also were included the plasmids p6200-47kb and p6411-9kb previously reported in Colombia. (B) BLASTn comparison of WGS assemblies of K. pneumoniae isolates from this study and the E. coli transconjugant C16-1 against the plasmid p6234-178kb. Regions Tra1 and Tra2 common to IncA/C2 plasmids are highlighted (Fernandez-Alarcon et al. 2011); the Tra2 region is disrupted by a variable region. Black circles correspond to the reference plasmids p6234-178kb (A) and pNDM-BJ01 (B), included as internal control.
Two other NDM-1-positive strains of Acinetobacter spp., isolated from other Colombian cities (Neiva and Pasto, 520 km away from each other, and 320 and 390 km away from Cali, respectively) were recently reported (Rojas et al. 2016). The blaNDM-1-positive plasmids from these strains, p6200-47kb and p6411-9kb were also found to be pNDM-BJ01-like, each with 99% nucleotide identity over 100% of the pNDM-BJ01 length (fig. 1A and supplementary data set 2, Supplementary Material online). In contrast to the similarity between the blaNDM-1-positive plasmids found in the three Colombian NDM-1-positive strains of Acinetobacter spp., the strains themselves were of different, unrelated sequence type (ST). The Neiva A. baumannii (harboring p6200-47kb) was ST322 and the Pasto A. nosocomialis (harboring p6411-9kb) was ST464 (Rojas et al. 2016), but the Cali A. baumannii isolate 19Aba78 belongs to ST239, as determined by the Pasteur MLST scheme for A. baumannii (Diancourt et al. 2010). These observations of the 19Aba78 isolate and the E. coli transconjugant C19-78 thus contribute further evidence of dissemination of closely related pNDM-BJ01-like plasmids among unrelated Acinetobacter spp. isolates, as has been reported in Asia and Latin America (Hu et al. 2012; Sun et al. 2013; Waterman et al. 2013; Zhang et al. 2013; Wang et al. 2014; Brovedan et al. 2015; Espinal et al. 2015; Feng et al. 2015; Jones et al. 2015; Li et al. 2015; Rojas et al. 2016), and also its capability to transfer to Enterobacteriaceae.
The blaNDM-1-Positive Plasmids Circulating in K. pneumoniae from Colombia Are Closely Related IncA/C2 Plasmids
Analysis of the WGS assemblies for the six K. pneumoniae isolates showed they all have sequences with high similarity to p6234-178kb (>99% nucleotide identity over >98% of the p6234-178kb length, fig. 1B and supplementary data set 2, Supplementary Material online), an IncA/C2 plasmid harboring blaNDM-1 from a recently reported K. pneumoniae isolated in Neiva (Rojas et al. 2016), which is 300 km away from Bogota in Colombia. It therefore seems likely that the blaNDM-1 gene is located on a closely related conjugative plasmid in all seven of these Colombian K. pneumoniae strains. Despite the plasmid similarities, the K. pneumoniae strains from Bogota (16Kpn1, 16Kpn2, 16Kpn3, 16Kpn5, 16Kpn7 and 16Kpn10) are all ST1043 (Escobar Perez et al. 2013), and not related to the ST392 of the K. pneumoniae isolate from Neiva (Rojas et al. 2016). The sequences that mapped to p6234-178kb, assembled into several contigs for each of the six Bogota K. pneumoniae strains (supplementary data set 1, Supplementary Material online), so the order of the contigs and their genomic location could not be determined. However, the WGS assembly of the NDM-1-positive E. coli transconjugant C16-1, obtained using K. pneumoniae strain 16Kpn1 as the donor, also showed 99% nucleotide identity over 98% of the length of p6234-178kb (fig. 1B and supplementary data set 2, Supplementary Material online), reinforcing the evidence that blaNDM-1 is likely to be located on such a related conjugative plasmid in the Bogota outbreak. To rule out the possibility that any related sequences were located in the recipient strain, all contigs that mapped to the chromosome of E. coli J53 were removed from the genome assembly of the transconjugant C16-1, and all remaining contigs were found to map to p6234-178kb, with no additional sequences relative to p6234-178kb (see supplementary fig. 1B), and only one confirmed difference due to the absence of an IS5075 element. Thus, this data demonstrates that highly related NDM-1-positive IncA/C2 conjugative plasmids are circulating among K. pneumoniae with different genetic backgrounds (ST392 and ST1043) in two distant cities in Colombia.
Providencia rettgeri from Colombia and Mexico Harbor blaNDM-1 in Different Not Reported Plasmids
Comparison of the WGS assemblies of the Colombian P. rettgeri isolates against the database of complete blaNDM-1-positive plasmid sequences (supplementary data set 2, Supplementary Material online), revealed that there were no matches over the full length of any known plasmid. The most closely related plasmid was the Inc group unclassified pPrY2001, from a Canadian P. rettgeri strain (Mataseje et al. 2014), with >99% nucleotide identity over 69–77% of the length of pPrY2001 (supplementary data set 2 and fig. S2, Supplementary Material online). By way of exception, 16Pre47 (isolated in Bogota) and RB152 (isolated in Bucaramanga) had significant nucleotide identity over only 22% and 15% of the length of pPrY2001, respectively (supplementary data set 2, Supplementary Material online). All four NDM-1-positive E. coli transconjugants derived from P. rettgeri donors, regardless of the relationship of the respective donor strain with the pPrY2001 plasmid, had sequences that matched to only a small section of pPrY2001 (<15% of the pPrY2001 length covered with >99% nucleotide identity). The main region of identity to pPrY2001 for all four transconjugants, as well as for 16Pre47 and RB152, was limited to the Tn125 remnant (see supplementary fig. S2, Supplementary Material online).
The P. rettgeri isolates from Colombia also had significant sequence matches with the IncA/C2 plasmid p6234-178kb (supplementary data set 2, Supplementary Material online), and in general to all the IncA/C2 NDM-positive plasmids, with >99% nucleotide identity over ∼50% of the length of IncA/C2 plasmids (supplementary data set 2, Supplementary Material online). However, the key characteristics of IncA/C2 plasmids were not found in the P. rettgeri genome assemblies, as neither the repA gene, which is highly conserved among IncA/C2 plasmids, nor any marker for known incompatibility groups (except 16Pre46 with an IncN match), could be identified (see supplementary fig. S3, Supplementary Material online). Furthermore, the corresponding representative E. coli transconjugant(s) of P. rettgeri isolates from Bogota and Bucaramanga differed in their sequence coverage of IncA/C2 plasmids. Providencia rettgeri isolates from Bogota had >99% nucleotide identity over ∼65% of the length of p6234-178kb (except 16Pre45 with identity to only 28% of the length), as did the corresponding representative E. coli transconjugant C16-36, suggesting the blaNDM-1-positive plasmids circulating in Bogota in P. rettgeri and in K. pneumoniae may be related. By contrast, although the P. rettgeri isolates from Bucaramanga had >99% nucleotide identity over ∼60% of the length of p6234-178kb (except RB152 with only 26% coverage), the corresponding representative E. coli NDM-1-positive transconjugants CRB151 and CRB152 mapped just to 9% of p6234-178kb (supplementary data set 2, Supplementary Material online), the 9% associated with just the Tn125 remnant. Thus, the high relatedness to the IncA/C2 plasmids observed in the donor strains but not the transconjugants, suggests that the Bucaramanga P. rettgeri isolates probably have the blaNDM-1 gene located in a plasmid that is unrelated to p6234-178kb, as well as a blaNDM--negative structure that is related to the blaNDM-1-positive plasmid circulating among P. rettgeri and K. pneumoniae in Bogota.
Interestingly, the Mexican P. rettgeri isolates (06-1617, 06-1619 and 06-1623) and the corresponding representative E. coli transconjugant (C06-1623) showed a high level of similarity to the Acinetobacter spp. pNDM-BJ01-like plasmids (>99% of nucleotide identity over 74–80% of the length of pNDM-BJ01; fig. 1A and supplementary data set 2, Supplementary Material online). This suggests that a pNDM-BJ01-like plasmid harboring blaNDM-1 is circulating among the P. rettgeri from Mexico, but with a truncated Tn125 and downstream deletion, relative to pNDM-BJ01 (fig. 1A). Additionally, the Mexican P. rettgeri isolates showed close relatedness to the pPrY2001 plasmid (>99% of nucleotide identity over 64–76% of the length of pPrY2001); however, the C06-1623 E. coli transconjugant did not. It mapped to only 11% of pPrY2001, which corresponded to the Tn125 remnant (see supplementary fig. S2, Supplementary Material online), suggesting that there is another blaNDM-negative genetic structure in the Mexican P. rettgeri that is related to pPrY2001. No known Inc group was identified in any of the genomes for the P. rettgeri isolates from Mexico (except 06-1623, with a match to the IncT group), as is also the case for pNDM-BJ01 (see supplementary fig. S3, Supplementary Material online), further supporting the evidence that blaNDM-1 is located on a pNDM-BJ01-like plasmid in these strains.
Phylogenetic Concordance with the Geographic Origin of P. rettgeri
To investigate the genetic relationship in the P. rettgeri, the major NDM-1-positive pathogen identified in the Colombian clinical surveillance (supplementary data set 1, Supplementary Material online), we used the WGS assemblies to build a phylogenetic tree based on the core genome SNPs among the 14 NDM-1-positive P. rettgeri isolates included in this study, and found that all but one of the isolates clustered according to the city of origin (fig. 2). The exceptional isolate, 16Pre45, clustered together with the strains from Bucaramanga, even though it was isolated in Bogota. Despite the genetic relationship, no epidemiological link with the Bucaramanga region was identified for the patient harboring 16Pre45 (supplementary data set 1, Supplementary Material online). These results suggest that blaNDM-1 dissemination in P. rettgeri in Colombia and Mexico is following a clonal behavior according to the geographic origin.
Fig. 2.
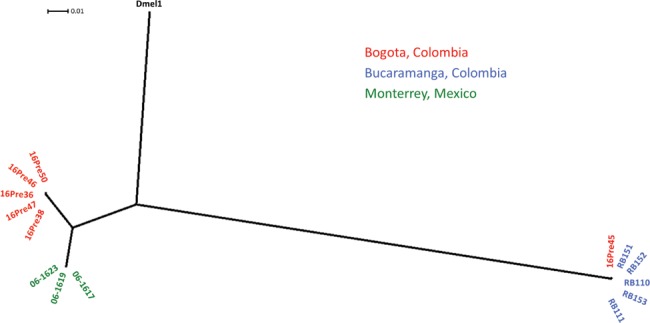
—Phylogenetic tree of Providencia rettgeri isolates based on core-genome SNPs. A Maximum Likelihood (ML) tree was built based on the SNPs in the core-genome assemblies of the NDM-1-positive P. rettgeri strains reported in this study (red, blue and green) with the P. rettgeri Dmel1 included as an outgroup control. Branch lengths are expressed in units of changes/nucleotide position (scale bar).
General Features of the Complete blaNDM-1-Positive Plasmids from Latin American P. rettgeri
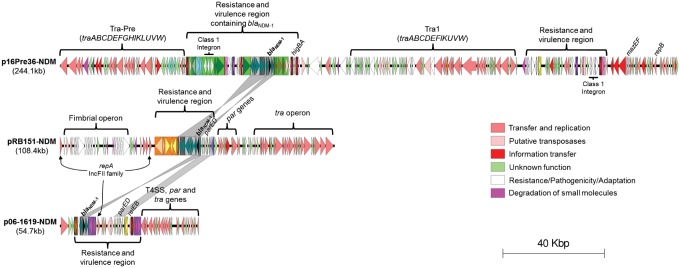
The complete sequences of plasmids from one representative isolate for each of the three P. rettgeri clusters (16Pre36 from Bogota, RB151 from Bucaramanga and 06-1619 from Monterrey), were obtained by PacBio sequencing. The number of plasmids in each strain varied: 16Pre36 had two plasmids (p16Pre36-NDM and p16Pre36-2), RB151 had one plasmid (pRB151-NDM) and 06-1619 had two plasmids (p06-1619-2 and p06-1619-NDM) (table 1). Among these representative P. rettgeri isolates, the blaNDM-1 gene was located on unrelated plasmids (fig. 3). None of the plasmids belonged to any reported incompatibility group using Carattoli et al. (2014) schemes (see supplementary fig. S3, Supplementary Material online), however, annotation of the plasmids pRB151-NDM and p06-1619-NDM, showed they have two and one repA genes, respectively (fig. 2), which are closely related to each other (>85% nucleotide identity over the full repA sequence). The putative plasmid replication proteins encoded by these repA genes each had a significant match to the IncFII RepA protein family in Pfam (Finn et al. 2016). However, these three repA genes showed a poor relation (<51% nucleotide identity over the full repA sequence) to the reported IncFII repA genes (Carattoli et al. 2014), and plasmids pRB151-NDM and p06-1619-NDM are not related to any reported IncFII plasmid (not shown). In p06-1619-NDM the repA gene is located inside a putative mobile element, which possibly brought repA from another plasmid (fig. 3).
Fig. 3.
—General description of the blaNDM-1-positive plasmids from three representative Providencia rettgeri isolates sequenced in this study, including regions encoding genes for transposition and for replication, and virulence and resistance regions. Plasmid p16Pre36-NDM has two tra regions, one reported only for a P. rettgeri isolate (Tra-Pre) and the other reported in different IncA/C2 plasmids (Tra1). Plasmids pRB151-NDM and p06-1619-NDM have putative repA genes belonging to the IncFII family. Insertion sequences or transposons are shown as rectangles containing their respective CDS for transposition and accessory genes. Gray bars between pairs of sequences indicates >90% nucleotide identity in a window of 400 bp. The scale bar indicates sequence length.
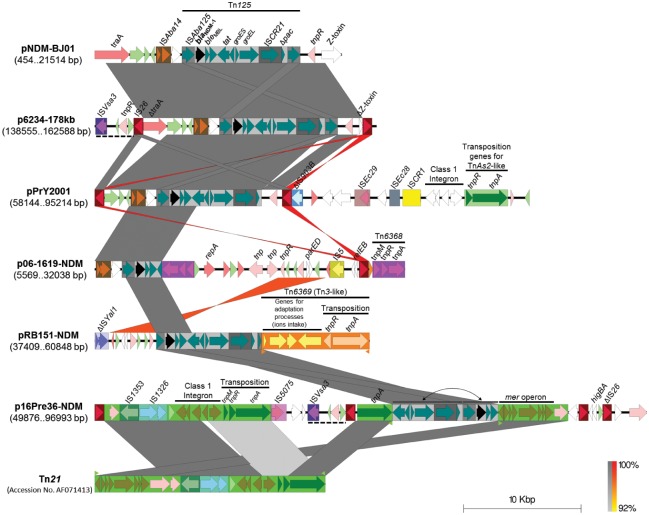
Plasmid p16Pre36-NDM was found to encode two different putative conjugative transfer-associated regions (fig. 3). One (described here as Tra-Pre), has so far only been reported in the blaNDM-1-positive plasmid pPrY2001; the other, is a common transfer-associated region of IncA/C2 plasmids described as Tra1 by Fernandez-Alarcon et al. (2011), which is frequently found in widely disseminated plasmids in a broad host range (Sekizuka et al. 2011; Doublet et al. 2012; Diene et al. 2013; Tijet et al. 2015; Wang et al. 2015; Wasyl et al. 2015; Rojas et al. 2016), including the IncA/C2 p6234-178kb-like blaNDM-1-positive plasmids in the Colombian K. pneumoniae strains (supplementary data set 1, Supplementary Material online). At 244,116 bp, p16Pre36-NDM is the largest and most variable of the blaNDM-1-positive plasmids sequenced in this study, and among the largest blaNDM-1-positive plasmids ever reported (supplementary data set 2, Supplementary Material online). It has two resistance regions each containing a toxin–antitoxin system and a class 1 integron. Both class 1 integrons have the genes dfrA1-aadA1-qacEΔ1-sul1 associated with resistance to quaternary ammonium compounds, aminoglycosides, sulphonamides and trimethoprim. This plasmid also carries the additional resistance genes aph(3′)-Ia, sul2 and tet(B). One of the resistance regions contains a Tn125 remnant (with its blaNDM-1 gene intact) inside a shuffled Tn21 element, with the two Tn21 inverted repeats flanking the Tn125 remnant (fig. 4). This shuffled Tn21 element has its typical components IS1353, IS1326, the mer operon and a class 1 integron (Liebert et al. 1999), but they are rearranged and the mer operon is separated from the rest of the Tn21 by the Tn125 remnant (fig. 4). Tn21-like elements are implicated in the global dissemination of antibiotic resistance genes among Enterobacteriaceae and Pseudomonas (Liebert et al. 1999) and have been reported to generate mosaic structures (Yurieva et al. 1997; Noguchi et al. 2000; Partridge et al. 2001; Valverde et al. 2006). Moreover, the ΔTn125 (having just one copy of the ISAba125 element) surrounding blaNDM-1 in p16Pre36-NDM has suffered a rearrangement that has not been previously reported: the genes tat-dct-groES-groEL-ISCR21-Δpac that are found upstream of the blaNDM-1 in p16Pre36-NDM, have always been previously reported downstream (fig. 4). These odd rearrangements of the ΔTn125 and the Tn21 in p16Pre36-NDM were confirmed by mapping of the PacBio reads to the assembled plasmid (no regions of low read coverage or quality were found that could suggest an assembly issue); by investigation of the MiSeq assembly (the same rearrangement was identified on a single contig, see supplementary fig. S4A, Supplementary Material online); and by using PCR to confirm the location of the ΔTn125 inside the Tn21 (see supplementary fig. S4B, Supplementary Material online). The entire ΔTn21 region in p16Pre36-NDM is flanked upstream and downstream by copies of IS26 (fig. 4), thus it is possible that this entire region of DNA may be mobilized as a composite transposon. IS26 intramolecular replicative transposition has been previously identified as the source of reorganization of plasmids carrying multidrug-resistant determinants as could have happened here (He et al. 2015).
Fig. 4.
—Comparison of variable blaNDM-1-containing regions from plasmids p16Pre36-NDM, pRB151-NDM, p06-1619-NDM, p6234-178kb, pPrY2001 and pNDM-BJ01. Insertion sequences or transposons are shown as rectangles containing their respective CDS for transposition and accessory genes in different colors. Outside orange and green triangles correspond to the inverted repeats of a putative Tn3-like (Tn6369) and a Tn21, respectively. The prototype sequence of the Tn21 was included (Liebert et al. 1999). Dashed lines indicate the 2,928-bp sequence containing ISVsa3 carried by p6234.178kb (once) and p16Pre36-NDM (twice). Gray and red (inverted matches) shading between pairs of sequences indicates >90% of nucleotide identity in a window of 400 bp. The scale bar indicates sequence length.
Compared with p16Pre36-NDM the pRB151-NDM (from Bucaramanga, Colombia) is a less complex plasmid, with a size of 108,417 bp. This is a novel plasmid backbone, unrelated to any previously reported (without any significant match against the NCBI nucleotide database), that encodes a putative conjugative transfer machinery, plasmid replication and partition proteins, a restriction-modification system and a putative fimbrial operon (fig. 3). It possesses only one resistance and virulence region, and that contains the blaNDM-1 gene as the plasmid’s only antibiotic resistance determinant (table 1). The variable region has a ΔTn125 harboring the blaNDM-1 with a typical structure except that the two flanking ISAba125 are both truncated (fig. 4). Downstream of the ΔTn125 in the resistance region is a novel transposon, registered as Tn6369 in the Tn Number Registry (Roberts et al. 2008), whilst upstream there is another putative mobile element (or its remains) encoding two putative transposon resolvases and a ParED toxin–antitoxin system that is also present in the p06-1619-NDM plasmid, plus an upstream ΔISYal1 (fig. 4).
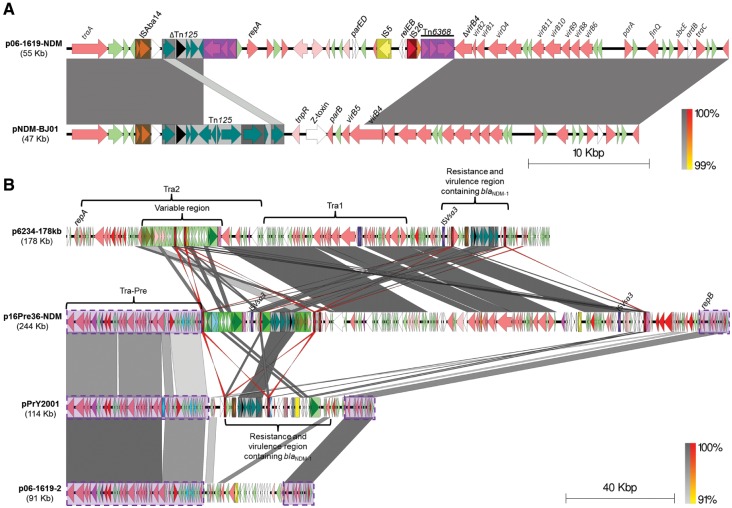
The p06-1619-NDM plasmid (from Mexico) has a size of 54,712 bp, and belongs to the conserved pNDM-BJ01-like family of plasmids (from Acinetobacter spp.), with the same backbone (99% nucleotide identity over 74% of the pNDM-BJ01 length, fig. 5A). However, it has a putative mobile genetic element (MGE) flanked by identical copies of a novel transposon with intact transposition genes, registered as Tn6368, that has inserted downstream of the blaNDM-1 gene. The insertion of this MGE, has truncated the Tn125 after the tat gene upstream (fig. 4), as well as deleted the genes parB and virB5 and truncated virB4 downstream, so truncating the T4SS locus (fig. 5A). These genes are implicated in the mating pair formation and DNA partitioning process (Christie et al. 2005; Schumacher and Funnell 2005; Kusiak et al. 2011). Their loss in this plasmid may not affect its conjugation capability given that in this study we were able to obtain an E. coli transconjugant from a Mexican P. rettgeri strain harboring the p06-1619-NDM plasmid (fig. 1A and supplementary fig. S1E, Supplementary Material online); although, it is also possible that the other plasmid in this strain, p06-1619-2, which contains the putative P. rettgeri conjugation machinery (Tra-Pre), could act as a helper for the conjugation process, as has been reported for other antibiotic resistance plasmids (Dery et al. 1997; Bennett 2008; Al-Marzooq et al. 2015). As well as the deletions described here, insertion of the putative novel mobile element has given the plasmid two toxin–antitoxin systems (parED and relEB), repA and the IS elements IS5 and IS26 (fig. 5A).
Fig. 5.
—BLASTn comparison of (A) pNDM-BJ01 with the related p06-1619-NDM plasmid from Providencia rettgeri, and (B) pPrY-like plasmids (p16Pre36-NDM, pPrY2001 and p06-1619-2) from P. rettgeri with the IncA/C2 related p6234-178kb plasmid reported in Klebsiella pneumoniae. Conserved pPrY-like region is highlighted in purple rectangles with dashed lines. Gray and red (inverted matches) shading between pairs of sequences indicate >90% of nucleotide identity in a window of 400 bp. The scale bar indicates sequence length.
Pairwise comparisons show there is no relationship between the three different P. rettgeri blaNDM-1-positive plasmids circulating in Colombia and Mexico, apart from the Tn125 remains and the presence of multiple copies of the IS26 element (fig. 3). Only the p16Pre36-NDM plasmid shows some relationship with p6234-178kb-like plasmids found in Colombian K. pneumoniae strains (supplementary data set 2, Supplementary Material online, and fig. 5B). Both p16Pre36-NDM and p6234-178kb share the same Tra1 region, although the Tn125 (harboring blaNDM-1) is located within a different genetic context (figs. 4 and 5B). These similarities explain the genetic relationship found among the blaNDM-1-positive IncA/C2 plasmids and the P. rettgeri from Bogota (supplementary data set 2, Supplementary Material online). However, p6234-178kb does not have the Tra-Pre region found in p16Pre36-NDM, and has a more complex antibiotic resistance gene profile (table 1) due to the presence of an additional class 1 integron containing most of those genes.
Surprisingly, despite no previously reported epidemiological connection, a genetic link between P. rettgeri strains from Colombia, Mexico and Canada was found through analysis of the plasmids the strains harbor. We found the putative P. rettgeri conjugation machinery (Tra-Pre) and some additional regions, first identified in pPrY2001 from the Canadian P. rettgeri strain 09ACRGNY2001, were also present in the blaNDM-negative p06-1619-2 plasmid from Mexico and in the blaNDM-1 positive p16Pre36-NDM plasmid from Bogota, Colombia (fig. 5B).
Genomic Epidemiology of the blaNDM-1-Positive Plasmids Circulating in Colombia and Mexico
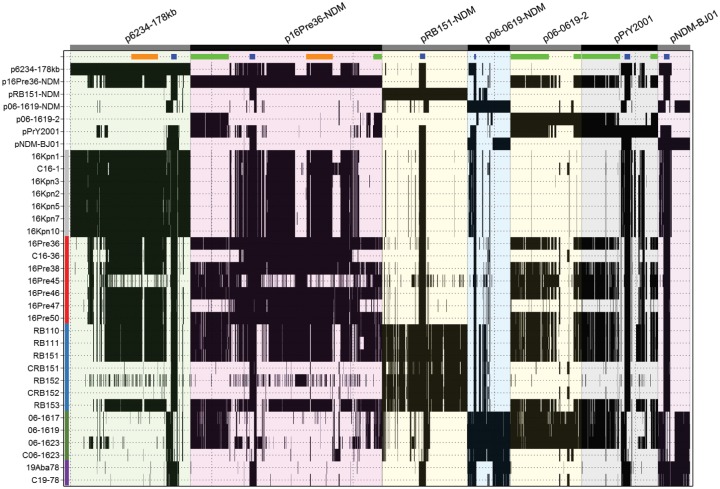
To gain a better understanding of the dissemination dynamics of blaNDM-1—positive plasmids in Latin America, the MiSeq sequencing reads for all isolates included in the study were mapped against the fully sequenced blaNDM-1-positive plasmids from the representative Colombian and Mexican strains, as well as the related blaNDM-1-positive plasmids pNDM-BJ01, pPrY2001 and the blaNDM-negative p06-1619-2 (fig. 6).
Fig. 6.
—Presence of blaNDM-1-plasmids in the clinical isolates and transconjugants. Complete sequence of blaNDM-1-plasmids circulating among Colombian and Mexican NDM-1-positive isolates and some related blaNDM-1-positive (pPrY2001 and pNDM-BJ01) and blaNDM-negative (p06-1619-2) plasmids are shown along the x axis. Black shading indicates a match of ≥90% nucleotide identity in a window of 300 bp, calculated by comparing the query sequence (x axis, reference plasmids) against the consensus from mapped reads for each strain (y axis). Horizontal orange and light green bars represent the Tra1 and pPrY-like regions, respectively; blue rectangles represent the Tn125 (or remnants) region in each plasmid. Vertical bars correspond to: gray for Klebsiella pneumoniae from Bogota (Colombia), red for Providencia rettgeri from Bogota (Colombia), blue for P. rettgeri from Bucaramanga (Colombia), green for P. rettgeri from Monterrey (Mexico), purple for Acinetobacter baumannii from Cali (Colombia). Simulated reads for the reference plasmids were included as an internal control.
The reads from four out of six P. rettgeri strains from Bogotá were found to map well over the entire sequence of p16Pre36-NDM (fig. 6), so are likely have blaNDM-1 in a plasmid closely related to p16Pre36-NDM. Of the remaining two P. rettgeri strains from Bogota, one, 16Pre47, lacked the Tra-Pre locus and the mapped reads covered only one section of the p16Pre36-NDM sequence, suggesting that 16Pre47 carries a much smaller variant of that plasmid; the other, 16Pre45, is positive for the Tra-Pre region, but has no reads mapping to several other regions of p16Pre36-NDM, so it is possible that it has a pPrY-like plasmid or an unrelated plasmid harboring the blaNDM-1 gene. Additionally, the reads for the E. coli transconjugant strain C16-36, generated from 16Pre36, did not map to the whole of the donor plasmid sequence, covering only a 138,178 bp section of p16Pre36-NDM, from position 67,813–206,190 bp (fig. 6 and supplementary fig. S1C, Supplementary Material online). This 138-kb region encodes the Tra1 region and ΔTn125 harboring the blaNDM-1 and has a 2,928-bp direct repeat sequence at each end (99% nucleotide identity to each other), which encodes ISVsa3 (known as an ISCR2-like element). The mapping profile (fig. 6) showed no C16-36 reads mapped to the Tra-Pre region common to pPrY2001 and its related plasmids (fig. 5B). Using primers specific to the Tra-Pre region a PCR product was generated for the donor strain 16Pre36 but not the transconjugant C16-36 (data not shown), confirming the absence of the Tra-Pre region in the transconjugant was due to transfer of only part of the donor plasmid and not a problem with the WGS data. Taken together, these data indicate that p16Pre36-NDM contains a smaller, self-mobilizing transposable element, 138 kb in size, that can be transferred via conjugation to a new host, possibly via the self-encoded Tra1 conjugative apparatus, common to IncA/C2 plasmids.
The sequencing reads from all five P. rettgeri isolates from Bucaramanga and the corresponding representative transconjugants CRB151 and CRB152, mapped to almost the entire pRB151-NDM sequence, suggesting that they harbor blaNDM-1 in a plasmid closely related to pRB151-NDM (fig. 6). Plasmid pRB151-NDM does not encode the Tra-Pre and Tra1 regions and the strain RB151 does not have additional plasmids, yet four out of five of the related P. rettgeri isolates from Bucaramanga (including RB151) had sequences that mapped to the pPrY2001-like and IncA/C2 Tra1 regions (fig. 6). Investigation of the complete RB151 genome sequence (Marquez-Ortiz et al. 2017) revealed the presence of a genomic island with a high degree of similarity to p16Pre36-NDM, including the Tra-Pre and Tra1 regions, which is chromosomally inserted in strain RB151, flanked by 14.6-kb direct repeats (99% nucleotide identity) (see supplementary fig. S5A, Supplementary Material online). This chromosomal insertion was confirmed by PCR using specific primers (see supplementary fig. S5B, Supplementary Material online).
The read mapping data also showed that all three of the P. rettgeri Mexican isolates have both p06-1619-NDM and p06-1619-2 plasmids, and the reads from the representative transconjugant C06-1623 showed good coverage over the entire length of p06-1619-NDM, confirming the presence of blaNDM-1 on this plasmid.
Thus, the conjugative transfer-associated region Tra-Pre was observed to be present in 12 out of 14 NDM-1-positive P. rettgeri isolates (fig. 6), from Colombia (Bogota and Bucaramanga) and Mexico. In addition to the first report in the Canadian P. rettgeri blaNDM-1-positive plasmid pPrY2001, this Tra-Pre region is also found in the partially sequenced genome of a NDM-positive P. rettgeri isolated in Israel, strain H1736 (Olaitan et al. 2015) (see supplementary fig. S2, Supplementary Material online). However, the partial assembly of the Israeli isolate prevented determination of whether or not the blaNDM-1 gene is located in the same plasmid as the Tra-Pre region. Together, these results validate a genetic link among epidemiologically unrelated isolates of NDM-1-positive P. rettgeri.
Discussion
In this study, we used WGS data to provide a high-resolution picture of blaNDM-1 dissemination in Latin America, which led us to interesting findings about the dissemination route of this gene between Enterobacteriaceae and Acinetobacter species. Acinetobacter spp. harboring blaNDM-1 in pNDM-BJ01-like plasmids are frequently detected all over the world (Feng et al. 2015; Fu et al. 2015); these (or NDM-positive isolates with blaNDM-1 genetic surroundings suggesting the presence of pNDM-BJ01-like plasmids) have even been found in Latin America (Waterman et al. 2013; Pasteran et al. 2014; Brovedan et al. 2015; Quinones et al. 2015; Montana et al. 2016; Rojas et al. 2016). Here, we report an A. baumannii clone isolated in Colombia that also harbors blaNDM-1 in a pNDM-BJ01-like plasmid. This finding supports observations of such a blaNDM-1-harboring plasmid present in Acinetobacter spp. of different genetic backgrounds. However, although the majority of plasmids harboring blaNDM in Acinetobacter spp. are pNDM-BJ01-like plasmids, and although there is some evidence to suggest that Acinetobacter spp. passed blaNDM on to the Enterobacteriaceae (Toleman et al. 2012), pNDM-BJ01-like plasmids do not seem to have good fitness in non-Acinetobacter bacteria, or at least in Enterobacteriaceae. To date, only one non-Acinetobacter harboring a pNDM-BJ01-like plasmid has been reported (Feng et al. 2015), whereas a plethora of Enterobacteriaceae hosting diverse, completely unrelated blaNDM-positive plasmids have been found (supplementary data set 2, Supplementary Material online). The mechanisms of blaNDM gene transmission from Acinetobacter spp. to Enterobacteriaceae are not yet understood.
In this study, we identified a second Enterobacteriaceae family member harboring a pNDM-BJ01-like plasmid (p06-1619-NDM), a P. rettgeri isolated in an outbreak in Mexico (Barrios et al. 2013). However, the p06-1619-NDM plasmid has suffered a major modification in the variable region, with the insertion of a previously unreported mobile element introducing two toxin–antitoxin systems (flanked by two novel Tn6368 transposons). It seems plausible that the toxin–antitoxin systems have generated a strong dependence on that plasmid as has been previously reported (Kamruzzaman et al. 2017), thereby avoiding transposition of blaNDM-1 to another more compatible plasmid and its subsequent elimination. Adding to the advantages conferred by the two addictive systems is the high selective pressure of the environment—an Intensive Care Unit—from which the Mexican P. rettgeri strains were isolated. As the other plasmid hosted by these isolates (p06-1619-2) does not have any resistance genes (table 1), selective pressure could force permanent residence of the pNDM-BJ01-like plasmid due to the conferred resistance to aminoglycosides and beta-lactams, including carbapenems.
Providencia species are frequently found in environmental settings, but are also opportunistic human pathogens, mainly as the causative agents of urinary tract infections (Wie 2015). They are not among the most significant or prevalent human threats, but recently they have been attracting interest due to increasing reports of P. rettgeri NDM-positive isolates found around the world (Barrios et al. 2013; Carvalho-Assef et al. 2013; Mataseje et al. 2014; Pollett et al. 2014; Tada et al. 2014; Carmo Junior et al. 2015; Manageiro et al. 2015; Nachimuthu et al. 2015; Wailan, Paterson, et al. 2016). Here, we also found P. rettgeri to be the most frequent bacteria harboring the blaNDM-1 gene in three hospitals at distant locations from each other in Colombia. Most of the cases correspond to outpatients, suggesting that NDM-1-positive P. rettgeri strains are present in the community in Colombia. In spite of the increase in NDM-positive P. rettgeri cases, only two completely sequenced blaNDM-1-positive plasmids are in the NCBI nucleotide database for P. rettgeri, both of which were isolated in Canada (supplementary data set 2, Supplementary Material online). Here, we report three additional unrelated complete plasmids hosted by P. rettgeri (two from Colombia and one from Mexico), providing more information to help elucidate blaNDM-1 dissemination in the Enterobacteriaceae.
Interestingly, despite the geographic distances between the sites of isolation of NDM-1-positive P. rettgeri strains and despite the very different structures of their blaNDM-1-positive plasmids, we found a common feature—a putative conjugative transfer region named here as Tra-Pre—that appears to be stable among P. rettgeri from different regions. Supporting this hypothesis of a common, stable feature for blaNDM-1-positive P. rettgeri, is the fact that this Tra-Pre region is also found in the partially sequenced genome of the NDM-1-positive P. rettgeri H1736, reported in Israel in 2011 (Olaitan et al. 2015).
The Tra-Pre-family plasmids harboring blaNDM-1 that are hosted by P. rettgeri (pPrY2001 and p16Pre36-NDM) are unrelated to the pNDM-BJ01-like plasmids from Acinetobacter species. Nevertheless, it is possible the blaNDM-1-positive Tra-Pre-encoding plasmids emerged in P. rettgeri through transposition of blaNDM-1 from a pNDM-BJ01-like plasmid (prior to its loss) to a more stable plasmid, as suggested by the coexistence of a blaNDM-negative plasmid containing the Tra-Pre region (p06-1619-2) and a pNDM-BJ01-like plasmid in the P. rettgeri from Mexico. This proposed mechanism is further supported by the presence of the Tra-Pre region in almost all P. rettgeri isolates in this study (12 out of 14), even isolates in which blaNDM-1 is found on an unrelated plasmid, for example in the P. rettgeri isolates from Bucaramanga, Colombia. The simultaneous detection in the Bucaramanga P. rettgeri isolates of a new plasmid harboring the blaNDM-1, and of a region in the bacterial chromosome encoding both the IncA/C2-related and Tra-Pre regions similar to those found in the blaNDM-1-positive plasmid circulating in Bogota, indicates how a possible transposition of the blaNDM-1 region to a new, different backbone may have occurred in this strain. Thus, our study supports the role of gene module transposition in the spread of blaNDM-1 among P. rettgeri clinical isolates, a role that has been identified as relevant to the evolution of blaNDM-1-positive plasmids (Khong et al. 2016).
We found a further interesting genetic link in the relationship between blaNDM-1-positive plasmids present in P. rettgeri (p16Pre36-NDM) and those found in K. pneumoniae (p6234-178kb). The isolates harboring p16Pre36-NDM and p6234-178kb were detected in the same Colombian hospital (supplementary data set 1, Supplementary Material online). Although p16Pre36-NDM and p6234-178kb cannot be classified in the same Inc group, they share a large region, commonly found in the IncA/C2 blaNDM-1-positive and negative plasmids from diverse Enterobacteriaceae. This region was also found to be inserted in the chromosome of the P. rettgeri isolates from Bucaramanga. These results suggest that the complex p16Pre36-NDM plasmid originated in P. rettgeri, through the co-integration of a pPrY2001-like plasmid with an acquired IncA/C2 broad host range plasmid from a different Enterobacteriaceae. This IncA/C2 plasmid may then have been transferred to (or from) a non-Providencia Enterobacteriaceae, such as the K. pneumoniae in this study. Conjugation of p16Pre36-NDM to E. coli J53 resulted in transfer of only part of the plasmid, a putative self-mobilizable ISVsa3 (an ISCR2-like element) composite transposon encoding the blaNDM-1 and Tra1 regions, but not the Tra-Pre region. The mapping data indicates that the clinical isolate 16Pre47 also only contains this ISVsa3 composite transposon, and is missing the remainder of p16Pre36-NDM, suggesting this Tra-Pre-negative strain 16Pre47 may have receive the ISVsa3 composite transposon from other P. rettgeri strain (through partial conjugation) or from a K. pneumoniae. This putative blaNDM-1-positive conjugative transposon may have derived from the p6234.178kb circulating in K. pneumoniae in Colombia prior to the isolation of the P. rettgeri, given their similarity and the presence of a closely related sequence containing ISVsa3 (2,928 bp) in both p6234.178kb and p16Pre36-NDM (figs. 4 and 5). Interestingly, this transposable element was found to be conserved (99% nucleotide identity over the 2,928 bp) in the genomes of a very wide range of bacteria, in a search against the NCBI database, and also ISVsa3-like elements has been recognized as key players in IncA/C plasmids evolution (Toleman and Walsh 2010). Therefore, the novel blaNDM-1-positive putative conjugative transposon identified in this study could facilitate broad dissemination of blaNDM-1 through transposition, conjugation and integration of the transferred circular intermediate into the host genome or to other plasmids via homologous recombination.
The low levels of similarity in the vicinity of Tn125 (or its remnants) between p16Pre36-NDM and p6234-178kb could be due to the later isolation of the P. rettgeri strain harboring p16Pre36-NDM. Although both the P. rettgeri and the K. pneumoniae strains were isolated at the same hospital (supplementary data set 1, Supplementary Material online), P. rettgeri harboring p16Pre36-NDM was isolated more than a year after the K. pneumoniae outbreak. The length of time the P. rettgeri were present in the hospital under selective pressure, could promote the genetic rearrangements observed in the p16Pre36-NDM plasmid. In an earlier isolate of K. pneumoniae, the blaNDM-1 surroundings on the p6234-178-kb plasmid are more closely related to those in Acinetobacter species.
Dissemination of the IncA/C2 p6234-178kb plasmid in different cities and in strains of different genetic backgrounds, is consistent with the globally observed trend for IncA/C2 (recently designated as IncC; Harmer et al. 2016) group plasmids, which are known to be associated with spread of multidrug resistance genes to different countries and different bacterial hosts (Fricke et al. 2009; Roy Chowdhury et al. 2011; Carattoli et al. 2012), meaning they post a significant risk to human health, particularly as they are capable of disseminating blaNDM-1. The importance of improving existing infection control measures, such as isolation of patients harboring resistant pathogens and hand hygiene, is further substantiated by our findings suggesting that some blaNDM-1-positive plasmids are likely to have originated by co-integration of less stable blaNDM-1-positive plasmids with more stable and disseminative plasmids in environmental bacteria. A case in point is found in the P. rettgeri isolates, in which the Tn125 (or its remains) may have transposed from pNDM-BJ01-like plasmids to pPrY2001-like or IncA/C2-related plasmids that could subsequently be transferred to other bacterial species, including more problematic non-Providencia Enterobacteriaceae. An important scenario where all these factors can be found simultaneously is in the mammalian gut where Enterobacteriaceae can thrive, aiding the inter-species and inter-genera dissemination of the NDM-1 antibiotic resistance gene among the bacterial community of the gut. Host-specific conditions, such as the inflammatory host response, can also boost horizontal gene transfer and hence microbiota evolution (Stecher et al. 2012), that may have led to the plasmid rearrangements observed here, probably under the selective pressure of the hospital environment. However, direct transfer from Acinetobacter spp. to non-Providencia Enterobacteriaceae cannot be ruled out, due to conjugation of pNDM-BJ01-like plasmids from Acinetobacter spp. to non-Providencia Enterobacteriaceae (mainly E. coli) has been demonstrated in this and other studies (Hu et al. 2012; Huang et al. 2015). However, more work needs to be done to better understand the genetic basis of the dissemination of blaNDM-1-positive plasmids in Latin America, by evaluating the stability, fitness cost and conjugation capability of pNDM-BJ01-like and pPrY2001-like plasmids to other Enterobacteriaceae.
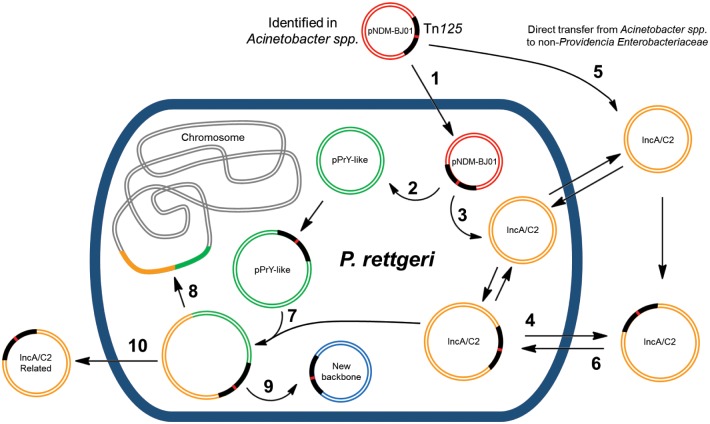
In our analysis of NDM-1-positive clinical isolates in Latin America, the high variability of blaNDM-1-positive plasmids present in different species, highlights that blaNDM-1-dissemination has not only followed a predominantly clonal evolution, but rather a Russian doll model (Sheppard et al. 2016). In this Russian doll model, a resistance gene such as blaNDM-1 resides on nested transmissible units and therefore can move through the environment at multiple different levels, that can be both coincident and independent of one another. For example, bacterial cell hosting resistance gene; plasmid within bacterial cell harboring resistance gene; mobile element within plasmid harboring resistance gene; and mobile element within mobile element harboring resistance gene, with the consequence that the resistance gene may move between plasmids within a bacterial cell via multiple mechanisms (Sheppard et al. 2016). In line with this model, different clones have acquired different blaNDM-1-positive plasmids and related strains have disseminated locally, as for example in the NDM-1 outbreaks in Colombia and Mexico (Barrios et al. 2013; Escobar Perez et al. 2013) and also the cases of the P. rettgeri isolated in Bogota and Bucaramanga (Colombia) in the recent surveillance study. These related strains acquired the blaNDM-1 from a variety of plasmids, such as IncA/C2-related or pPrY2001-like plasmids, that in turn received blaNDM-1 from plasmid co-integration or transposition from another plasmid, or from Acinetobacter spp. pNDM-BJ01-like plasmids in an initial dissemination stage. In our Russian doll model, P. rettgeri plays an important role as a reservoir of blaNDM-1 available for transmission into highly disseminative plasmids due to its high recombination capability supported by the high plasmid variability found in this species (fig. 7). In this study, the presence of pNDM-BJ01-like, Tra-Pre-encoding and IncA/C2-related plasmids or genetic structures in P. rettgeri, and their relationship with the plasmids present in K. pneumoniae and Acinetobacter species, illustrates the evolution route of blaNDM-1-positive plasmids in Latin America, where P. rettgeri appears to be crucial for blaNDM-1 transmission from Acinetobacter spp. to Enterobacteriaceae.
Fig. 7.
—Possible roles of Providencia rettgeri in blaNDM-1-plasmids evolution in Latin America. In an initial stage pNDM-BJ01-like plasmids are acquired from Acinetobacter spp. (−1). Shortly after, blaNDM-1 is transposed to pPrY-like plasmids (from P. rettgeri circulation; −2) or IncA/C2 plasmids (from Klebsiella pneumoniae, Escherichia coli or other Enterobacteriaceae; −3) via Tn125 transposition or by mean of other mobile genetic elements surrounding the Tn125 (or its remnants). IncA/C2 blaNDM-1-plasmids could be transferred to a broad bacteria host range (−4). It is also possible that a non-Providencia Enterobacteriaceae could capture a pNDM-BJ01-like plasmid and transposes Tn125 to a broad host range IncA/C2 plasmid (−5); later this IncA/C2 blaNDM-1-plasmid could be conjugated to P. rettgeri (−6). An interesting finding of the present study is the generation in P. rettgeri of new plasmids by mean of co-integration of pPrY-like plasmids and IncA/C2 plasmids (−7). These chimeric structures can also be transposed to the P. rettgeri chromosome (−8). The Tn125 (or its remnants) could be transposed to new plasmid backbones with possible implications upon its dissemination (−9). Additionally, by mean of partial conjugation could be disseminated IncA/C2-related (repA negative) blaNDM-1-plasmids (−10).
Taken together, these findings expose the role of microorganisms such as P. rettgeri, that generally are not the target of public health surveillance systems, in the dissemination and storage of resistance genes, highlighting the importance of more comprehensive studies, which do not merely focus on the most frequently occurring pathogens but also encompass the resistance determinants and their mobilization machinery.
Supplementary Material
supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
We gratefully acknowledge financial support from the Departamento Administrativo de Ciencia, Tecnología e Innovación, Colciencias (grant number 130871250819 and Fellowship 567-2012 to R.A.M.); Vicerrectoría de Investigaciones, Universidad El Bosque (grant number PCI-2012-330) and University of Technology Sydney. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author Contributions
R.A.M., J.E. and N.K.P. designed research; R.A.M., L.H. and N.K.P. performed research; N.O., C.D., U.G.R., J.S.S., B.E.C., E.M.S., M.B., M.V.M., J.E.C. and A.V. contributed new reagents/analytic tools; R.A.M., J.E. and N.K.P. analyzed data; R.A.M., I.G.C., N.V., J.E. and N.K.P. conceived the study; and R.A.M. and N.K.P. wrote the paper.
Literature Cited
- Al-Marzooq F, Mohd Yusof MY, Tay ST.. 2015. Molecular analysis of antibiotic resistance determinants and plasmids in Malaysian isolates of multidrug resistant Klebsiella pneumoniae. PLoS One 10:e0133654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA.. 2011. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics 12:402.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ.. 1990. Basic local alignment search tool. J Mol Biol. 215:403–410. [DOI] [PubMed] [Google Scholar]
- Bankevich A, et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 19:455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios H, et al. 2013. Isolation of carbapenem-resistant NDM-1-positive Providencia rettgeri in Mexico. J Antimicrob Chemother. 68:1934–1936. [DOI] [PubMed] [Google Scholar]
- Bennett PM. 2008. Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br J Pharmacol. 153(Suppl 1):S347–S357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W.. 2011. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27:578–579. [DOI] [PubMed] [Google Scholar]
- Boetzer M, Pirovano W.. 2012. Toward almost closed genomes with GapFiller. Genome Biol. 13:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovedan M, et al. 2015. Complete sequence of a blaNDM-1-harboring plasmid in an Acinetobacter bereziniae clinical strain isolated in Argentina. Antimicrob Agents Chemother. 59:6667–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A, Villa L, Poirel L, Bonnin RA, Nordmann P.. 2012. Evolution of IncA/C blaCMY-2-carrying plasmids by acquisition of the blaNDM-1 carbapenemase gene. Antimicrob Agents Chemother. 56:783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A, et al. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 58:3895–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo Junior NV, et al. 2015. First report of a NDM-producing Providencia rettgeri strain in the state of Sao Paulo. Braz J Infect Dis. 19:675–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Assef AP, et al. 2013. Isolation of NDM-producing Providencia rettgeri in Brazil. J Antimicrob Chemother. 68:2956–2957. [DOI] [PubMed] [Google Scholar]
- Carver TJ, et al. 2005. ACT: the Artemis Comparison Tool. Bioinformatics 21:3422–3423. [DOI] [PubMed] [Google Scholar]
- CDC 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep. 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- Chain PS, et al. 2009. Genomics. Genome project standards in a new era of sequencing. Science 326:236–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, et al. 2015. NDM-1 encoded by a pNDM-BJ01-like plasmid p3SP-NDM in clinical Enterobacter aerogenes. Front Microbiol. 6:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin CS, et al. 2013. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10:563–569. [DOI] [PubMed] [Google Scholar]
- Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E.. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu Rev Microbiol. 59:451–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M, Dzamba M, Lister D, Ilie L, Brudno M.. 2011. SHRiMP2: sensitive yet practical SHort Read Mapping. Bioinformatics 27:1011–1012. [DOI] [PubMed] [Google Scholar]
- Dery KJ, et al. 1997. Characterization of the replication and mobilization regions of the multiresistance Klebsiella pneumoniae plasmid pJHCMW1. Plasmid 38:97–105. [DOI] [PubMed] [Google Scholar]
- Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S.. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5:e10034.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diene SM, et al. 2013. The rhizome of the multidrug-resistant Enterobacter aerogenes genome reveals how new "killer bugs" are created because of a sympatric lifestyle. Mol Biol Evol. 30:369–383. [DOI] [PubMed] [Google Scholar]
- Doublet B, et al. 2012. Complete nucleotide sequence of the multidrug resistance IncA/C plasmid pR55 from Klebsiella pneumoniae isolated in 1969. J Antimicrob Chemother. 67:2354–2360. [DOI] [PubMed] [Google Scholar]
- Escobar Perez JA, et al. 2013. Outbreak of NDM-1-producing Klebsiella pneumoniae in a neonatal unit in Colombia. Antimicrob Agents Chemother. 57:1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal P, et al. 2011. Dissemination of an NDM-2-producing Acinetobacter baumannii clone in an Israeli rehabilitation center. Antimicrob Agents Chemother. 55:5396–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinal P, et al. 2015. Identification of NDM-1 in a putatively novel Acinetobacter species ("NB14") closely related to Acinetobacter pittii. Antimicrob Agents Chemother. 59:6657–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, et al. 2015. Coexistence of a novel KPC-2-encoding MDR plasmid and an NDM-1-encoding pNDM-HN380-like plasmid in a clinical isolate of Citrobacter freundii. J Antimicrob Chemother. 70:2987–2991. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alarcon C, Singer RS, Johnson TJ.. 2011. Comparative genomics of multidrug resistance-encoding IncA/C plasmids from commensal and pathogenic Escherichia coli from multiple animal sources. PLoS One 6:e23415.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, et al. 2016. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44:D279–D285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke WF, et al. 2009. Comparative genomics of the IncA/C multidrug resistance plasmid family. J Bacteriol. 191:4750–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, et al. 2015. Spread of a common blaNDM-1-carrying plasmid among diverse Acinetobacter species. Infect Genet Evol. 32:30–33. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Hamidian M, Ambrose SJ, Hall RM.. 2016. Destabilization of IncA and IncC plasmids by SGI1 and SGI2 type Salmonella genomic islands. Plasmid 87–88:51–57. [DOI] [PubMed] [Google Scholar]
- He S, et al. 2015. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. MBio 6:e00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, et al. 2012. Novel plasmid and its variant harboring both a blaNDM-1 gene and type IV secretion system in clinical isolates of Acinetobacter lwoffii. Antimicrob Agents Chemother. 56:1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TW, et al. 2015. Effective transfer of a 47 kb NDM-1-positive plasmid among Acinetobacter species. J Antimicrob Chemother. 70:2734–2738. [DOI] [PubMed] [Google Scholar]
- Huang W, Li L, Myers JR, Marth GT.. 2012. ART: a next-generation sequencing read simulator. Bioinformatics 28:593–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M, et al. 2015. Circlator: automated circularization of genome assemblies using long sequencing reads. Genome Biol. 16:294.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Scornavacca C.. 2012. Dendroscope 3: an interactive tool for rooted phylogenetic trees and networks. Syst Biol. 61:1061–1067. [DOI] [PubMed] [Google Scholar]
- Johnson AP, Woodford N.. 2013. Global spread of antibiotic resistance: the example of New Delhi metallo-beta-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol. 62:499–513. [DOI] [PubMed] [Google Scholar]
- Jones LS, et al. 2015. Characterization of plasmids in extensively drug-resistant Acinetobacter strains isolated in India and Pakistan. Antimicrob Agents Chemother. 59:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamruzzaman M, Shoma S, Thomas CM, Partridge SR, Iredell JR.. 2017. Plasmid interference for curing antibiotic resistance plasmids in vivo. PLoS One 12:e0172913.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong WX, et al. 2016. Local transmission and global dissemination of New Delhi Metallo-Beta-Lactamase (NDM): a whole genome analysis. BMC Genomics 17:452.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusiak M, Gapczynska A, Plochocka D, Thomas CM, Jagura-Burdzy G.. 2011. Binding and spreading of ParB on DNA determine its biological function in Pseudomonas aeruginosa. J Bacteriol. 193:3342–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos EV, et al. 2014. Impact of carbapenem resistance on clinical and economic outcomes among patients with Acinetobacter baumannii infection in Colombia. Clin Microbiol Infect. 20:174–180. [DOI] [PubMed] [Google Scholar]
- Li P, et al. 2015. Acinetobacter calcoaceticus from a fatal case of pneumonia harboring blaNDM-1 on a widely distributed plasmid. BMC Infect Dis. 15:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebert CA, Hall RM, Summers AO.. 1999. Transposon Tn21, flagship of the floating genome. Microbiol Mol Biol Rev. 63:507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loytynoja A. 2014. Phylogeny-aware alignment with PRANK. Methods Mol Biol. 1079:155–170. [DOI] [PubMed] [Google Scholar]
- Manageiro V, et al. 2015. Draft genome sequence of the first NDM-1-producing Providencia stuartii strain isolated in Portugal. Genome Announc. 3:e01077–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, et al. 2008. Urinary tract infections in a South American population: dynamic spread of class 1 integrons and multidrug resistance by homologous and site-specific recombination. J Clin Microbiol. 46:3417–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Ortiz RA, et al. 2017. First complete Providencia rettgeri genome sequence, the NDM-1-producing clinical strain RB151. Genome Announc. 5:e01472–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17:10–12. [Google Scholar]
- Mataseje LF, et al. 2014. Complete sequences of a novel blaNDM-1-harbouring plasmid from Providencia rettgeri and an FII-type plasmid from Klebsiella pneumoniae identified in Canada. J Antimicrob Chemother. 69:637–642. [DOI] [PubMed] [Google Scholar]
- Milne I, et al. 2013. Using Tablet for visual exploration of second-generation sequencing data. Brief Bioinform. 14:193–202. [DOI] [PubMed] [Google Scholar]
- Montana S, et al. 2016. Presence of New Delhi metallo-beta-lactamase gene (NDM-1) in a clinical isolate of Acinetobacter junii in Argentina. New Microbes New Infect. 11:43–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachimuthu R, et al. 2015. Characterization of carbapenem-resistant Gram-negative bacteria from Tamil Nadu. J Chemother. 28:371–374. [DOI] [PubMed] [Google Scholar]
- Noguchi N, Katayama J, Sasatsu M.. 2000. A transposon carrying the gene mphB for macrolide 2′-phosphotransferase II. FEMS Microbiol Lett. 192:175–178. [DOI] [PubMed] [Google Scholar]
- Olaitan AO, Diene SM, Assous MV, Rolain JM.. 2015. Genomic plasticity of multidrug-resistant NDM-1 positive clinical isolate of Providencia rettgeri. Genome Biol Evol. 8:723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, et al. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palzkill T. 2013. Metallo-beta-lactamase structure and function. Ann N Y Acad Sci. 1277:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge SR, Iredell JR.. 2012. Genetic contexts of blaNDM-1. Antimicrob Agents Chemother. 56:6065–6067. author reply 6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge SR, Recchia GD, Stokes HW, Hall RM.. 2001. Family of class 1 integrons related to In4 from Tn1696. Antimicrob Agents Chemother. 45:3014–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasteran F, et al. 2014. Emergence of genetically unrelated NDM-1-producing Acinetobacter pittii strains in Paraguay. J Antimicrob Chemother. 69:2575–2578. [DOI] [PubMed] [Google Scholar]
- Poirel L, Dortet L, Bernabeu S, Nordmann P.. 2011. Genetic features of blaNDM-1-positive Enterobacteriaceae. Antimicrob Agents Chemother. 55:5403–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollett S, et al. 2014. Phenotypic and molecular characteristics of carbapenem-resistant Enterobacteriaceae in a health care system in Los Angeles, California, from 2011 to 2013. J Clin Microbiol. 52:4003–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabaker K, Weinstein RA.. 2011. Trends in antimicrobial resistance in intensive care units in the United States. Curr Opin Crit Care 17:472–479. [DOI] [PubMed] [Google Scholar]
- Quinones D, et al. 2015. High prevalence of blaOXA-23 in Acinetobacter spp. and detection of blaNDM-1 in A. soli in Cuba: report from National Surveillance Program (2010-2012). New Microbes New Infect. 7:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhomberg PR, Jones RN.. 2009. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: a 10-year experience in the United States (1999-2008). Diagn Microbiol Infect Dis. 65:414–426. [DOI] [PubMed] [Google Scholar]
- Roberts AP, et al. 2008. Revised nomenclature for transposable genetic elements. Plasmid 60:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas LJ, et al. 2016. Initial assessment of the molecular epidemiology of blaNDM-1 in Colombia. Antimicrob Agents Chemother. 60:4346–4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Chowdhury P, et al. 2011. Dissemination of multiple drug resistance genes by class 1 integrons in Klebsiella pneumoniae isolates from four countries: a comparative study. Antimicrob Agents Chemother. 55:3140–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford K, et al. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. [DOI] [PubMed] [Google Scholar]
- Schmieder R, Edwards R.. 2011. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27:863–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Funnell BE.. 2005. Structures of ParB bound to DNA reveal mechanism of partition complex formation. Nature 438:516–519. [DOI] [PubMed] [Google Scholar]
- Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. [DOI] [PubMed] [Google Scholar]
- Sekizuka T, et al. 2011. Complete sequencing of the blaNDM-1-positive IncA/C plasmid from Escherichia coli ST38 isolate suggests a possible origin from plant pathogens. PLoS One 6:e25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AE, et al. 2016. Nested Russian doll-like genetic mobility drives rapid dissemination of the carbapenem resistance gene blaKPC. Antimicrob Agents Chemother. 60:3767–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siguier P, Varani A, Perochon J, Chandler M.. 2012. Exploring bacterial insertion sequences with ISfinder: objectives, uses, and future developments. Methods Mol Biol. 859:91–103. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, et al. 2012. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc Natl Acad Sci U S A. 109:1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Petty NK, Beatson SA.. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. 2013. Complete genome sequence of an Acinetobacter strain harboring the NDM-1 Gene. Genome Announc. 1:e0002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, et al. 2014. NDM-1 Metallo-beta-Lactamase and ArmA 16S rRNA methylase producing Providencia rettgeri clinical isolates in Nepal. BMC Infect Dis. 14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera G, Castresana J.. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 56:564–577. [DOI] [PubMed] [Google Scholar]
- Tijet N, Richardson D, MacMullin G, Patel SN, Melano RG.. 2015. Characterization of multiple NDM-1-producing Enterobacteriaceae isolates from the same patient. Antimicrob Agents Chemother. 59:3648–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleman MA, Spencer J, Jones L, Walsh TR.. 2012. blaNDM-1 is a chimera likely constructed in Acinetobacter baumannii. Antimicrob Agents Chemother. 56:2773–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toleman MA, Walsh TR.. 2010. ISCR elements are key players in IncA/C plasmid evolution. Antimicrob Agents Chemother. 54:3534; author reply 3534.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde A, et al. 2006. In117, an unusual In0-like class 1 integron containing CR1 and blaCTX-M-2 and associated with a Tn21-like element. Antimicrob Agents Chemother. 50:799–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn PJ, Dautzenberg MJ, Oostdijk EA.. 2011. Recent trends in antibiotic resistance in European ICUs. Curr Opin Crit Care 17:658–665. [DOI] [PubMed] [Google Scholar]
- Wailan AM, Paterson DL, et al. 2016. Genomic characteristics of NDM-producing Enterobacteriaceae isolates in Australia and their blaNDM genetic contexts. Antimicrob Agents Chemother. 60:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wailan AM, Sidjabat HE, et al. 2016. Mechanisms involved in acquisition of blaNDM genes by IncA/C2 and IncFIIY Plasmids. Antimicrob Agents Chemother. 60:4082–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, et al. 2015. IncA/C plasmids harboured in serious multidrug-resistant Vibrio cholerae serogroup O139 strains in China. Int J Antimicrob Agents 45:249–254. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. 2014. Complete genome sequence of Acinetobacter baumannii ZW85-1. Genome Announc. 2:e01083–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasyl D, Kern-Zdanowicz I, Domanska-Blicharz K, Zajac M, Hoszowski A.. 2015. High-level fluoroquinolone resistant Salmonella enterica serovar Kentucky ST198 epidemic clone with IncA/C conjugative plasmid carrying blaCTX-M-25 gene. Vet Microbiol. 175:85–91. [DOI] [PubMed] [Google Scholar]
- Waterman PE, et al. 2013. Bacterial peritonitis due to Acinetobacter baumannii sequence type 25 with plasmid-borne new delhi metallo-beta-lactamase in Honduras. Antimicrob Agents Chemother. 57:4584–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wie SH. 2015. Clinical significance of Providencia bacteremia or bacteriuria. Korean J Intern Med. 30:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford N, Turton JF, Livermore DM.. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 35:736–755. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization. [Google Scholar]
- Yong D, et al. 2009. Characterization of a new metallo-beta-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 53:5046–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurieva O, et al. 1997. Intercontinental spread of promiscuous mercury-resistance transposons in environmental bacteria. Mol Microbiol. 24:321–329. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, et al. 2013. Complete sequence of the blaNDM-1-carrying plasmid pNDM-AB from Acinetobacter baumannii of food animal origin. J Antimicrob Chemother. 68:1681–1682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.