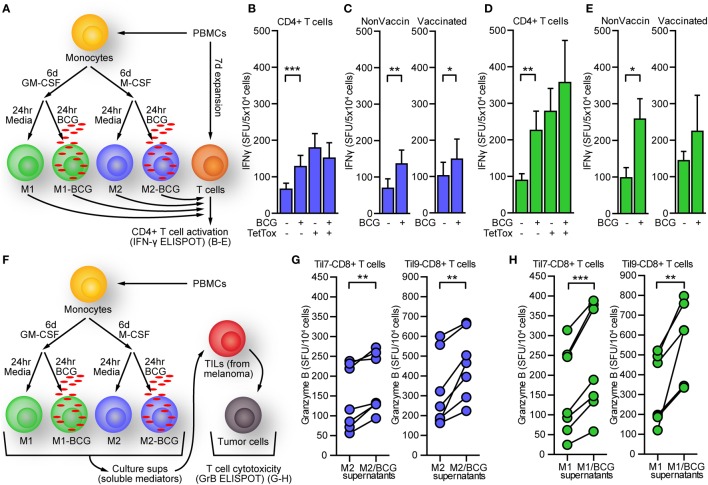
Figure 4.
Bacillus Calmette–Guérin (BCG)-treated macrophages induce greater activation on T cells in the absence of antigen, and their supernatant can condition tumor-infiltrating lymphocytes (TILs) to improve antitumor immunity. (A) In vitro polarization, infection, and enzyme-linked immunoSpots (ELISPOTs). Macrophages were polarized, infected, and harvested as explained in the Section “Materials and Methods.” Meanwhile, another fraction of peripheral blood mononuclear cells (PBMCs) was cultured for 7 days in 10% fetal calf serum media-RPMI (in the presence of tetanus toxoid) and subsequently enriched for CD4+ T cells by negative selection. These T cells were assayed in ELISPOT experiments with macrophages. (B) M2-BCG (blue bars) increased significantly the frequency of interferon gamma (IFN-γ)-producing autologous T cells in an antigen non-specific manner. (C) The response was observed regardless of BCG vaccine status (*p < 0.05, **p < 0.01, Wilcoxon signed rank test). (D) M1-BCG (green bars) induced an even greater enhancement on the frequency of IFN-γ-producing autologous T cells, an effect not favored by previous BCG vaccination (E). (F) Supernatants from polarized, BCG-infected macrophages were used to condition TILs before targeting autologous melanoma cells. (G) Supernatants from M2-BCG enhanced granzyme B (GrB) release from TILs in response to autologous tumor cells. (H) Likewise, M1-BCG supernatants also increased GrB release. Number of GrB SFU from independent experiments with supernatant sets from seven different donors are shown as the mean ± SEM. Results with two different pairs of TILs and autologous tumor cells are shown (**p < 0.01, ***p < 0.001, Wilcoxon signed rank test). SFU, spot forming units. Error bars indicate SEM.