Figure 1.
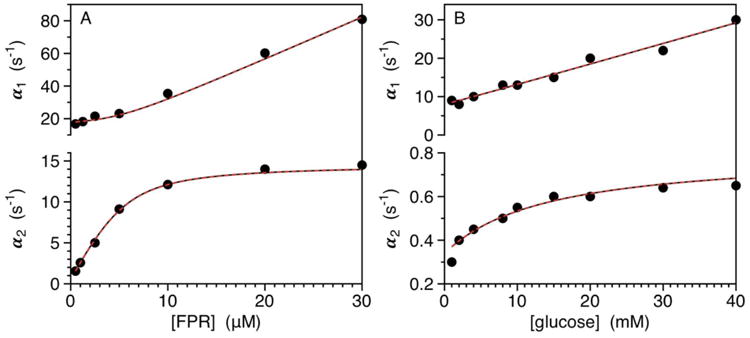
A-B. (A) Rates of relaxation measured for FPR binding to thrombin (50 nM) under experimental conditions of: 50 mM Tris, 200 mM ChCl, 0.1% PEG8000, pH 8 at 10 °C. FPR is in large excess (>10:1) even at the lowest concentration used, so the system is studied under pseudo-first order conditions of ligand. The fast relaxation (top panel) increases linearly with FPR at high concentrations reflecting the binding interaction. The slow relaxation (bottom panel) increases hyperbolically and monitors the conformational transition that either precedes (CS) or follows (IF) the binding step. Interpretation of the data in terms of IF (red curves) yields best-fit parameter values: , , k23=12±1 s-1, k32=2.6±0.1 s-1. The mathematically identical interpretation in terms of CS (black curves) returns the best-fit parameter values: , , k12=14.6±0.5 s-1, k21=3.1±0.3 s-1. The fits are identical because of the complete equivalence of IF and CS according to eqs. 4a-e and Scheme 4 (see also Scheme 5). (B) Rates of relaxation for glucose binding to glucokinase (5-10 μM), taken from ref (20). As in the case of FPR binding to thrombin (panel A), the ligand is in large excess (>100:1) even at the lowest concentrations used and two relaxations monitor binding (top panel) and conformational transitions (bottom panel). The original report used the rapid equilibrium approximation to assign the mechanism as IF. Interpretation of the data in terms of IF (red curves) yields best-fit parameter values: , , k23=0.44±0.02 s-1, k32=0.36±0.01 s-1. The mathematically equivalent interpretation in terms of CS (black curves) returns best-fit parameter values: , k12=0.80±0.02 s-1, k21=7.2±0.2 s-1. The equivalence between CS and IF is based on eqs. 4a-e and Scheme 4 (see also Scheme 6).
