Figure 5.
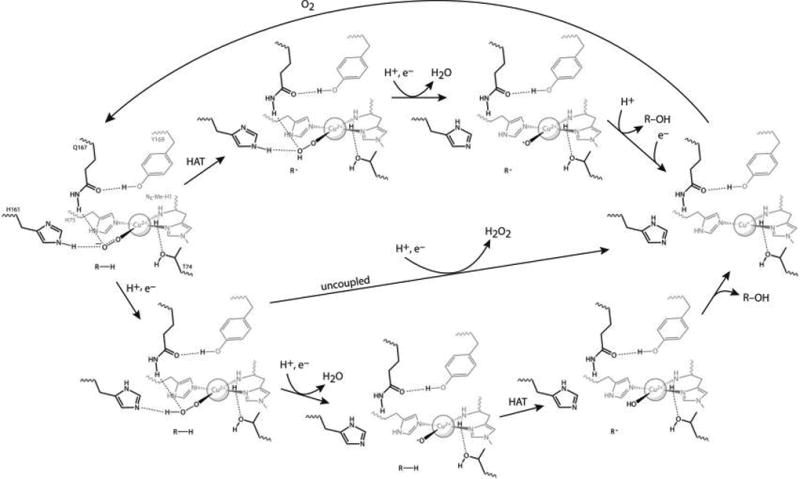
Proposed MtPMO3* reaction mechanisms. Both begin at left with the generally agreed upon Cu(II)–superoxo species that forms upon O2 binding to MtPMO3*-Cu(I). The top half follows one possible mechanism based on hydrogen atom transfer (HAT) by the superoxo intermediate, followed by reduction and cleavage of the distal O atom of the hydroperoxo intermediate to form water and an oxyl intermediate that undergoes radical rebound with the substrate. After substrate release, a one-electron reduction returns the Cu(I) enzyme (at right). The bottom half follows a possible mechanism utilizing an oxyl species for HAT, which is formed by reducing and cleaving the terminal O atom of the O2 adduct to form water. Uncoupling of oxygen activation from substrate hydroxylation could follow the middle pathway that produces peroxide. The release of superoxide from the H161 variants shows that H161 plays a proton transfer role to the Cu(II)-superoxo species, at least in the uncoupled reaction.
