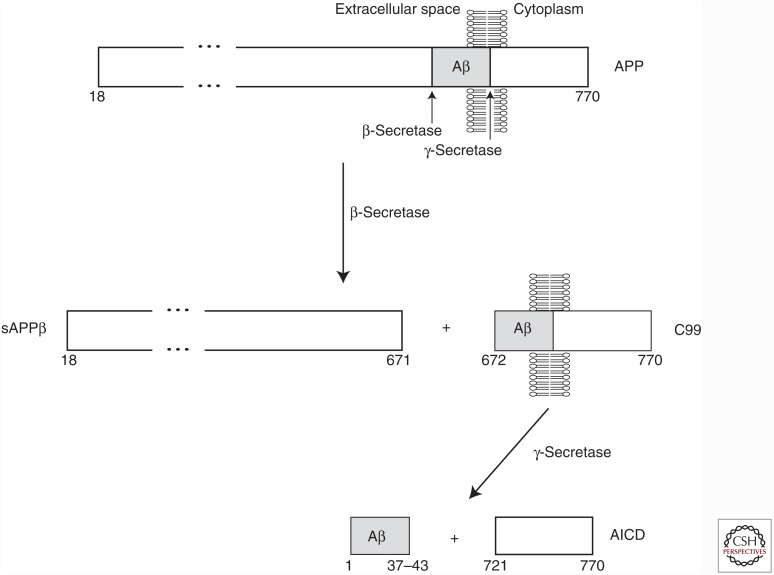
Figure 1.
Generation of β-amyloid (Aβ) peptide via endoproteolytic cleavage of amyloid precursor protein (APP). The mature form of the longest isoform of human APP (following removal of an N-terminal signal peptide) spans residues 18–770. In the amyloidogenic pathway, cleavage of the APP extracellular domain by β-secretase produces a secreted fragment termed sAPPβ and a membrane-embedded C-terminal fragment called C99. Cleavage of C99 by γ-secretase produces the APP intracellular domain (AICD) and the Aβ peptide. Cleavage of APP by γ-secretase is heterogeneous, resulting in the generation of Aβ peptides that vary in length between 37 and 43 residues.