Abstract
Background
Limited data exist on drug resistance and antiretroviral treatment (ART) outcomes in HIV-1 infected children in West Africa. We determined the prevalence of baseline resistance and correlates of virologic failure (VF) in a cohort of ART naïve HIV-1 infected children <10 years of age initiating ART in Mali.
Methods
Reverse transcriptase and protease genes were sequenced at baseline (before ART) and at 6 months. Resistance was defined according to the Stanford HIV Genotypic Resistance database. VF was defined as viral load ≥1000 copies/mL after 6 months of ART. Logistic regression was used to evaluate factors associated with VF or death >1 month after enrollment. Post hoc, antiretroviral concentrations were assayed on baseline samples of participants with baseline resistance.
Results
120 children with a median age 2.6 years (IQR: 1.6–5.0) were included. 88% reported no PMTCT exposure. At baseline, 27 (23%), 4 (3%), and none had NNRTI, NRTI or PI resistance, respectively. 39 (33%) developed VF and 4 died >1 month post-ART initiation. In multivariable analyses, poor adherence (OR 6.1, p=0.001), baseline NNRTI resistance among children receiving NNRTI-based ART (OR 22.9, p<0.001) and PI-based ART initiation among children without baseline NNRTI resistance (OR 5.8, p=0.018) were significantly associated with VF/death. Ten (38%) with baseline resistance had detectable levels of nevirapine or efavirenz at baseline; 7 were currently breastfeeding but only 2 reported maternal antiretroviral use.
Conclusions
Baseline NNRTI resistance was common in children without reported NNRTI exposure and was associated with increased risk of treatment failure. Detectable NNRTI concentrations were present despite few reports of maternal/infant antiretroviral use.
Keywords: Pediatrics, antiretroviral therapy, HIV drug resistance, treatment failure
Introduction
Before 2010, World Health Organization (WHO) guidelines recommended non-nucleoside reverse transcriptase inhibitor (NNRTI)-based first-line regimens for all HIV-infected children meeting criteria for antiretroviral therapy (ART) initiation.1 In response to data showing that children with prior nevirapine (NVP) exposure had higher rates of treatment failure with NNRTI-based versus protease inhibitor (PI)-based ART, the guidelines were updated in 2010 to include PI-based regimens as preferred first-line ART in children <24 months of age with prior NNRTI exposure via prevention of mother to child transmission (PMTCT).2,3 Subsequent evidence favoring PI-based first-line ART even in children without prior NVP exposure informed the 2013 WHO recommendation of PI-based first-line ART in all children <36 months of age irrespective of their NNRTI exposure history.4,5
While access to ART in resource-limited nations has greatly improved, new challenges include sustainability of effective treatment and management of therapeutic failure. Treatment failure in HIV-infected children in resource-limited nations, estimated to be 26%–50%, is thought to be due to a combination of poor adherence, inadequate drug levels and acquired resistance from maternal ART exposure.6–10 Additionally, HIV-infected breastfeeding infants of ART treated mothers are at increased risk of multi-class resistance.11 Baseline-NNRTI resistance rates are high in programs with high PMTCT coverage but these rates drop precipitously after 18 months of age, suggesting that baseline resistance is due mainly to NNRTI exposure through PMTCT and not transmitted resistance.12 Based on these data, and the undesirable attributes of PI-based regimens, including poor palatability and cold chain requirements, current guidelines recommend NNRTI-based first-line ART for children ≥36 months of age. Baseline resistance testing is not recommended due to its limited availability and dearth of knowledge about its drivers and impact on outcomes. This study aimed to determine the prevalence of baseline NNRTI-resistance and its association with virologic failure in HIV-infected children initiating first-line ART in Bamako, Mali.
Materials and Methods
Between November 2010 - February 2013, HIV-infected children <10 years of age initiating ART were recruited from the Gabriel Touré Hospital outpatient pediatric HIV clinic and one satellite clinic in Bamako, Mali. The clinics provide ART, prophylaxis and management of opportunistic infections, CD4 count and viral load (VL) testing, if available, free of charge to all children ≤15 years. The Institutional Review Boards of University of Bamako School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, and University of Washington approved this study.
Study design and patients
Participants who had previously received ART (except for PMTCT) were excluded. Written, informed consent was obtained from the participant’s guardian. Participants were evaluated at baseline (prior to ART initiation) and after 6 months of ART. Demographic and clinical information were obtained at both visits. VL and CD4 counts at both visits, if available, were extracted from the medical record. VL testing was performed as part of the study at the 6-month visit. At both visits, blood samples were obtained and plasma was stored at −20°C for future resistance testing. During the study period, first-line ART in Mali mirrored WHO 2010 guidelines: lopinavir/ritonavir + two nucleoside reverse transcriptase inhibitors (NRTIs) for children <24 months and NNRTI-based regimens for children ≥24 months, irrespective of PMTCT exposure history. Medication adherence and toxicity data are collected monthly following ART initiation as standard of care; these data were extracted from the medical record.
Laboratory assays
Six-month VL measurements were performed at the Centre de Recherche et Formation at the University of Bamako using the Abbott Molecular m2000 RealTime Assay. Genotypic resistance testing was performed on baseline samples and for participants with VL ≥1000 copies/mL at the 6-month visit at the Pitié-Salpêtrière Hospital in Paris, France. An in-house sanger population sequencing was performed using the ANRS (French Agency for HIV Research and Hepatitis) technique (http://www.hivfrenchresistance.org/ANRS). Automated nucleic acid extraction was done with NucliSENS® easyMAG (Biomerieux, France) for HIV RNA isolation.
Two PCR runs (first PCR and Nested PCR) were performed on a 9700PE to generate an amplicon containing protease and reverse transcriptase genes. PCR products were sequenced using BigDye Terminators v3.1 on an ABI 3730 Genetic Analyzer (Applied Biosystems, Foster City California, USA). Consensus sequences were aligned and edited by using SmartGene HIV software (Innovation Park, Lausanne, Switzerland). Resistance was defined as presence of mutations associated with intermediate or high-level drug resistance, using the Stanford algorithm version 7.0 (http://hivdb.stanford.edu/).
Post hoc, assessment for unreported exposure to antiretrovirals (ARVs) prior to ART initiation was performed on baseline samples from participants with baseline NNRTI resistance, including the following locally available ARVs: efavirenz, nevirapine, zidovudine, abacavir, emtricitabine, lamivudine, didanosine, stavudine, lopinavir, and ritonavir (lower limit of detection <10ng/mL). Plasma concentrations were determined using liquid chromatography coupled with tandem mass spectrometry (Waters Acquity UPLC® and Acquity TQD®, Waters Corp, Milford, MA, USA) in the Department of Pharmacology at the Bichat-Cl Bernard hospital in Paris, France.13
Statistical methods
Descriptive statistics were calculated for variables of interest, including cross-tabulation with respect to the main endpoint of virologic failure (defined as viral load ≥1000 copies/mL at/after 6 months) or death >1 month after enrollment, and to the main variable of interest (baseline NNRTI resistance). To evaluate bivariate associations with the endpoint and with NNRTI resistance, Fisher’s exact test was used for categorical variables, and Wilcoxon-Mann-Whitney test for quantitative variables.
To further evaluate the effect of baseline NNRTI resistance upon the main endpoint, multivariable logistic regression was used, adjusting for basic demographics (gender, age at enrollment, distance from clinic) as well as key variables suspected a-priori of affecting the endpoint (adherence and presence of an NNRTI in the initial regimen). An interaction term between use of NNRTI-based ART and baseline NNRTI resistance was added because the risk of failure was assumed to vary by ART regimen. Distance of residence from clinic was categorized as <5 kilometers (km), 5–10km and >10km. Poor adherence was defined as report of missed or late doses in more than 1 out of the 6 month study period. Given the small sample size and prevalence of missing data for some variables, no automated model-selection methods were used. Sensitivity analyses included excluding early death from the endpoint and expanding the definition of failure to viral load ≥40 copies/mL and ≥400 copies/mL. A p-value of <0.05 was considered significant.
To compare the rates of virologic, immunologic and clinical failure, participants were classified as meeting immunologic and/or clinical failure criteria based on 2013 WHO guidelines.5 Immunologic failure was defined as 6 month CD4 count <750 cells/mm3 for children <1 year, <500 cells/mm3 for children ≥1 and <2 years, <200 cells/mm3 for children ≥2 and <5 years, and <100 cells/mm3 for children ≥5 years. Clinical failure was defined as WHO stage 3 or 4 at 6 months after having a lower WHO stage at baseline. The analytical dataset was derived from the raw data using SAS 9.4 (SAS Institute, Cary NC) and R 3.2.1 (R Foundation for Statistical Computing, Vienna). All subsequent analysis was performed using R, versions 3.2.1–3.3.1.
Results
One hundred fifty ART naïve participants were enrolled; baseline resistance testing was available for 139 (93%). Five participants died within 1 month of ART initiation, 8 were lost to follow-up and 6 did not have a 6-month blood sample, leaving 120 participants to include in the final analysis. As there were no significant differences for any of the baseline characteristics between participants included and excluded in the final model, only baseline characteristics of the 120 participants included in the final model are shown in Table 1.
Table 1.
Baseline characteristics of HIV+ participants (N=120) with complete 6-month followup
| Characteristics | N (%) |
|---|---|
| Age (years), median (IQR) | 2.6 (1.6–5.0) |
| Female | 48 (40%) |
| No antenatal PMTCT exposure | 106 (88%) |
| Breastfed1 | 109 (91%) |
| Breastfeeding duration (months), median (IQR) | 18 (7–24) |
| Maternal ARV post-delivery | 16 (13%) |
| Mother alive | 104 (87%) |
| First born child | 15 (13%) |
| WHO Stage 3 or 4 | 103 (86%) |
| CD4 count (cells/mm3), N=109, median (IQR) | 683 (413–1,043) |
| Viral load (log copies/mL), N=50, median (IQR) | 5.8 (4.4–6.2) |
| HIV-1 Subtype2 | |
| CRF02_AG | 91 (76%) |
| CRF06_cpx | 13 (11%) |
| A1 | 6 (5%) |
| Other3 | 10 (8%) |
| ART regimen initiated on study | |
| NNRTI-based | 72 (60%) |
| PI-based | 48 (40%) |
| NRTI combination initiated on study | |
| ABC + 3TC | 30 (25%) |
| AZT + 3TC | 84 (70%) |
| D4T + 3TC | 6 (5%) |
Includes exclusive and mixed breastfeeding. Data missing for 6 participants.
Data missing for 2 participants
Includes A2 (1), C (1), CRF09_cpx (1), CRF27_cpx (1), B (2), G (2), Non-B Indeterminate (2)
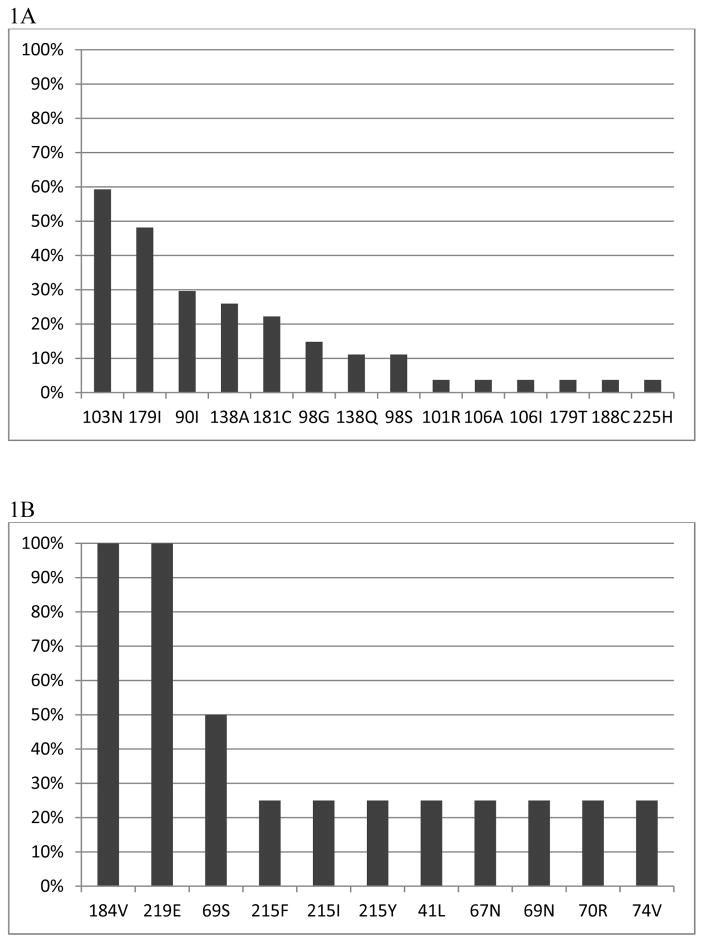
At baseline, 27/120 (23%) participants had NNRTI resistance and 4 (3%) had NRTI resistance (Figure 1). None had PI resistance and only 2 had >1 NNRTI resistance mutation. The most common NNRTI mutations were K103N (16) and Y181C (6) found in 22/27 (81%) participants with any NNRTI resistance. Based on the Stanford algorithm, 23 (19%) had high-level resistance to nevirapine and 16 (13%) had high-level resistance to efavirenz. All 4 with NRTI resistance also had NNRTI resistance. Among participants aged ≥36 months (n=56) at ART initiation, 9 (16%) had baseline NNRTI resistance and 3 (5%) had NRTI resistance.
Figure 1.
Frequency of NNRTI-and NRTI-resistance associated mutations at baseline. 1A: Frequency of NNRTI-resistance associated mutations in 27/120 (23%) participants with NNRTI-resistance associated mutations at baseline. 1B: Frequency of NRTI-resistance associated mutations in 4/120 (3%) participants with NRTI-resistance associated mutations at baseline.
There was no association between gender, WHO stage, breastfeeding status, PMTCT exposure or maternal postnatal ART with baseline NNRTI resistance. Participants with baseline NNRTI resistance were younger than those without, median age 2.4 vs. 2.8 years (p=0.02) with 29% of all participants <2 years of age and 19% of all ≥2 years harboring baseline NNRTI resistance (p=0.19). Baseline NNRTI resistance was similar (22%) among participants who initiated NNRTI- or PI-based ART.
The majority of participants initiated NNRTI-based ART (Table 1); 4/45 participants <2 years of age (9%) and 68/75 (91%) ≥2 years. Two (1.6%) participants switched from NNRTI-based to PI-based regimens, 9 (7.5%) switched from PI-based to NNRTI-based regimens and one switched from NNRTI-based to a triple NRTI-based regimen. The most commonly cited reason for PI to NNRTI switch was medication intolerance. Rates of poor adherence were similar with NNRTI-based versus PI-based ART (21%).
Four (3%) participants died after >1 month of ART; 3 of unknown causes and 1 from tuberculosis. At 6 months, 43 (36%) had the composite outcome of virologic failure or death (Table 2). In participants with and without the composite outcome, there was no significant difference in proportion of participants aged <2 vs. ≥2 years (p=0.46). Five participants had clinical failure; only 2 of these experienced virologic failure. Only two participants experienced immunologic failure.
Table 2.
Unadjusted associations between baseline characteristics and virologic failure or death1 at 6-months
| Virologic failure2 (n=43) | No virologic failure (n=77) | p-value | |
|---|---|---|---|
|
| |||
| N (%) | N (%) | ||
| Age (years), median (IQR) | 2.4 (1.5–5.2) | 2.8 (1.6–5.0) | 0.58 |
| Female | 16 (37%) | 32 (42%) | 0.70 |
| Baseline WHO stage 3 or 4 | 38 (88%) | 65 (84%) | 0.79 |
| Antenatal PMTCT exposure | 3 (7%) | 0 (0%) | 0.79 |
| Maternal ARV postdelivery | 10 (23%) | 6 (8%) | 0.04 |
| Any breastfeeding3 | 40 (93%) | 69 (90%) | 0.74 |
| Poor adherence | 16 (37%) | 9 (12%) | 0.002 |
| Baseline NNRTI-resistance | 18 (42%) | 9 (12%) | <0.001 |
| ART regimen initiated on study | |||
| NNRTI-based | 23 (53%) | 49 (64%) | 0.33 |
| PI-based | 20 (47%) | 28 (36%) | 0.33 |
Death occurring at least 1 month after ART initiation
Also includes 4 deaths occurring at least 1 month after ART initiation.
Includes exclusive and mixed breastfeeding.
Resistance testing was performed in 37/39 participants with virologic failure (median VL 4.8 log copies/mL [IQR: 3.9–5.52]); samples for two participants were not amplifiable. Of the 37 participants tested, 26 (70%) had both NNRTI and NRTI resistance; three (8%) additional participants had NRTI resistance only. The most common NNRTI mutations at 6 months remained K103N (16) and Y181C (8), constituting 22/26 (85%) participants with NNRTI resistance. 25/37 (67%) participants tested had resistance to nevirapine, 17 (46%) had resistance to efavirenz and 3 (8%) had resistance to rilpivirine. 17/26 (65%) participants with 6 month resistance also harbored baseline NNRTI resistance. None of the 20 failing participants receiving PI-based ART developed PI resistance after 6 months of ART.
Results of the univariate analysis are presented in Table 2. Maternal postnatal ART was associated with virologic failure (p=0.04); however, it was not included in the final model because data were missing for 11 mothers and adding it to the model did not change the main effect. In multivariable analyses, poor adherence, baseline NNRTI resistance among children who initiated NNRTI-based ART (OR 22.9, p<0.001) and initiation of PI-based ART among children without baseline NNRTI resistance (OR 5.8, p=0.018) were significantly associated with virologic failure or death (Table 3). Children with baseline NNRTI resistance who initiated PI-based therapy had higher odds of virologic failure or death but this was not statistically significant. Excluding early death from the endpoint did not significantly change the point estimates (data not shown). Using a viral load cut-off of ≥400 copies/mL, the associations seen in the main model remained significant but the observed point estimates were attenuated (data not shown).
Table 3.
Adjusted associations between baseline characteristics and virologic failure or death1 at 6-months
| Odds ratio | CI | p-value | |
|---|---|---|---|
| Age (per year) | 1.23 | 0.94, 1.60 | 0.13 |
| Gender (male vs. female) | 0.95 | 0.37, 2.41 | 0.91 |
| Residence distance from clinic | |||
| ≤5km | 2.07 | 0.59, 7.31 | 0.26 |
| 5–10km | Ref | -- | -- |
| >10km | 0.88 | 0.31, 2.49 | 0.81 |
| Presence of baseline NNRTI resistance | |||
| Initial NNRTI-based ART | 22.9 | 4.74, 110.3 | <0.001 |
| Initial PI-based ART | 2.68 | 0.01, 0.93 | 0.19 |
| Initial PI-based ART without baseline NNRTI resistance | 5.75 | 1.36, 24.4 | 0.018 |
| Poor adherence | 6.05 | 2.01, 18.2 | 0.001 |
Death occurring at least 1 month after ART initiation
ARV concentrations were assayed in 26/27 participants with baseline NNRTI resistance. Ten (38%) had detectable nevirapine (n=8; median 661ng/mL [IQR: 512–1050]) or efavirenz (n=2; median 179 ng/mL [IQR 144–213]). Compared to those without detectable ARVs, those with detectable levels were younger (median age 1.33 [IQR: 1.06–2.5] vs. 2.56 years [IQR: 1.65–3.34] p=0.07) and more likely to be currently breastfeeding (70% vs. 12.5%, p=0.009). Of the participants who had previously breastfed, the time since weaning for those with detectable drug(s) was similar to those without detectable drug (range: 1.12–1.76 years vs. 0.13–3.08 years). There was no association between maternal receipt of peri- or post-partum ARVs or infant receipt of ARVs for PMTCT and presence of detectable drug at baseline. Of the 7 participants who had detectable drug at baseline and were currently breastfeeding, 5 had no reported history of maternal antiretroviral administration.
Discussion
We found a high rate of drug resistance in HIV-infected children initiating first-line ART in Mali, with NNRTI resistance present at baseline in 23%. In addition, among children receiving NNRTI-based ART, baseline NNRTI resistance was associated with increased odds of virologic failure following six months of first-line ART.
In a cohort of newly diagnosed HIV-infected children <2 years of age in South Africa, 46% had baseline NNRTI resistance and 13% had baseline NRTI resistance.12 After stratifying by PMTCT exposure, 24% and 11% of PMTCT-unexposed children harbored baseline NNRTI and NRTI mutations, respectively, rates comparable to our results in predominantly PMTCT-unexposed and older children. When stratifying by age < or ≥2 years, we found baseline NNRTI resistance in 29% and 19%, respectively, and NRTI resistance in 2% and 4%, respectively. The high rates of baseline NNRTI resistance in our cohort are noteworthy since prior surveillance studies of baseline resistance in ART naïve adults in Mali demonstrated only a 1.5–4% and 1–4% prevalence of baseline NNRTI and NRTI resistance, respectively.14,15 This suggests that perinatal transmission of resistant virus is not the dominant source of the baseline resistance in our study. NNRTI resistance secondary to single dose nevirapine (sdNVP) for PMTCT typically decays by 24 months.16–18 The fact that we saw a high rate of baseline NNRTI resistance in children ≥36 months strongly suggests that PMTCT exposure alone does not explain our results. ARV exposure outside PMTCT is the other plausible explanation for some of the resistance observed in our study. In fact, 37% of children with baseline NNRTI resistance had detectable levels of nevirapine and/or efavirenz at baseline. In many of these children, breastfeeding was the likely source of ARV exposure. However, only 70% of children with detectable drug were currently breastfeeding and, among them, only 20% reported maternal postpartum ART. Studies of breastfeeding infants of ART-treated mothers have demonstrated detectable levels of NRTIs and NNRTIs.19–24 Tenofovir, lamivudine and efavirenz are detectable in breastfeeding infants at 6 months but by 12 months of age are below the limit of quantification despite continued exposure via breastfeeding, likely due to maturation of the infant metabolic clearance system.23 In our study, half of the children with detectable drug were either not currently breastfeeding or reported no maternal post-partum ART, and all were >12 months of age, raising the possibility of surreptitious or other unreported exposure to antiretroviral drugs, as has been described in adults.25
Regardless of the source of the resistant virus, it is concerning that 9% of our cohort who would qualify for NNRTI-based first-line ART in the current WHO guidelines harbored NNRTI-resistance. Our study employed population consensus sequencing capable of detecting variants that constitute at least 20% of the viral pool, and children with low-level resistance according to the Stanford Algorithm were not considered resistant in our analysis. Prior studies have shown that allele-specific PCR can detect up to 10% more resistance-associated mutations than population sequencing.17 Thus, we would have likely detected more baseline NNRTI-resistance had we used ultrasensitive PCR or included low-level resistance in our analysis.
K103N is the predominant NNRTI-resistance associated mutation detected in women exposed to sdNVP26,27; however, children are more likely to have Y181C detected following sdNVP.17,18,28 In contrast to prior studies, K103N was the predominant baseline NNRTI-resistance mutation in our cohort. This may reflect different resistance mechanisms and suggests that some of the baseline resistance in our cohort was transmitted.
Prior studies have reported virologic failure rates of 13–20% within 1 year of ART initiation.29–31 The virologic failure rate of 36% observed in our study was higher, though we followed participants for only 6 months. The failure rate in our study is similar to rates observed in studies that included a longer duration of ART (>1 year), and is likely related to the higher baseline resistance in our cohort.32 The prevalence of resistance associated mutations at virologic failure in our cohort is similar to prior reported rates of 52–97%.29,32
There are few studies examining the association between pre-treatment resistance and virologic failure during ART. In Uganda, children with NNRTI resistance mutations prior to ART initiation were more likely to develop virologic failure compared to those without, but the difference was not statistically significant.31 In our cohort, children with baseline NNRTI resistance mutations who initiated NNRTI-based ART were 23 times more likely to have virologic failure compared to children without NNRTI resistance. As expected, there was a weaker association between baseline NNRTI resistance and virologic failure during PI-based therapy. Since the age-regimen distribution reflects a policy similar to the current WHO guidelines, the increased odds is an indicator of the potential risk increase for patients ≥3 years, due to harboring NNRTI resistance. Interestingly, among children without baseline NNRTI resistance, initiating PI-based therapy was significantly associated with virologic failure. This finding persisted after controlling for adherence, though caregiver-reported adherence tends to be a poor indicator of true adherence33.
Our study had several limitations. Very few children had baseline viral load and CD4 data, which precluded their inclusion in the final model. Because the timing of HIV infection was unknown and reporting of PMTCT and postpartum maternal ART history was incomplete, we are unable to differentiate the contributions of NNRTI exposure versus transmitted resistance to our results. The fact that 37% of children with baseline NNRTI resistance had detectable levels of nevirapine and/or efavirenz suggests that some of the resistance was due to NNRTI exposure. As we did not measure drug levels in children without baseline NNRTI resistance, we cannot ascertain the association between drug detection and baseline resistance. Further, maternal ART and breastfeeding histories were sometimes provided by other caregivers, which limited our ability to verify the details. Nevertheless, several of our findings suggest unreported antiretroviral drug exposure.
In conclusion, a significant proportion of ART-naïve HIV-infected children in our Malian cohort harbored NNRTI-resistant virus that greatly increased the risk of virologic failure of NNRTI-based treatment. In addition, over a third of the children with baseline NNRTI resistance had detectable nevirapine and/or efavirenz despite few reports of maternal ART or PMTCT, and no reports of infant ART. A considerable number of children ≥36 months harbored baseline NNRTI-resistant virus, suggesting that resistance testing may be important whenever NNRTI-based treatment is being contemplated for this age group.
Acknowledgments
CSC and APO performed the data analysis. All authors contributed to writing the draft. We would like to acknowledge the children and caregivers who participated in this study.
Sources of funding: This study was funded by the Thrasher Research Fund Early Career Award, Lurie Children’s Hospital Colman Family Grant and Seattle Children’s Hospital Faculty Research Support Fund. D.B.F. was funded by the Agence Nationale de Recherches sur le SIDA et les Hépatites virales. C.S.C. and M.S. were also supported by the NIH through a Fogarty International Center grant D43TW007995 with R.L.M. as PI. This publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
Footnotes
Conflicts of interest: E.G.C. owns stock and stock options in Abbvie/Abbott Labs. B.T. has served as a consultant to ViiV, Pfizer, Janssen, GlaxoSmithKline and Gilead, and received research support to Northwestern University from ViiV and Pfizer. A.G.M. has received travel grants, consultancy fees, honoraria, or study grants from Bristol-Myers-Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. G.P. has received travel grants, consultancy fees, honoraria, or study grants from Bristol-Myers-Squibb, Gilead Sciences, Janssen, and Merck and ViiV Healthcare.
References
- 1.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access. [accessed September 24 2015];Recommendations for a public health approach. 2006 http://www.who.int/hiv/pub/guidelines/art/en/ [PubMed]
- 2.World Health Organization. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. [accessed September 24 2015];Recommendations for a public health approach 2010 revision. http://www.who.int/hiv/pub/paediatric/infants2010/en/ [PubMed]
- 3.Palumbo P, Lindsey JC, Hughes MD, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. The New England journal of medicine. 2010;363(16):1510–20. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violari A, Lindsey JC, Hughes MD, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. The New England journal of medicine. 2012;366(25):2380–9. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [accessed September 24 2015];Recommendations for a Public Health Approach. 2013 Jun; http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [PubMed]
- 6.Germanaud D, Derache A, Traore M, et al. Level of viral load and antiretroviral resistance after 6 months of non-nucleoside reverse transcriptase inhibitor first-line treatment in HIV-1-infected children in Mali. The Journal of antimicrobial chemotherapy. 2010;65(1):118–24. doi: 10.1093/jac/dkp412. [DOI] [PubMed] [Google Scholar]
- 7.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. Journal of acquired immune deficiency syndromes (1999) 2007;45(3):311–7. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adje-Toure C, Hanson DL, Talla-Nzussouo N, et al. Virologic and immunologic response to antiretroviral therapy and predictors of HIV type 1 drug resistance in children receiving treatment in Abidjan, Cote d’Ivoire. AIDS research and human retroviruses. 2008;24(7):911–7. doi: 10.1089/aid.2007.0264. [DOI] [PubMed] [Google Scholar]
- 9.Fassinou P, Elenga N, Rouet F, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Cote d’Ivoire. AIDS (London, England) 2004;18(14):1905–13. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- 10.Reddi A, Leeper SC. Antiretroviral therapy adherence in children: outcomes from Africa. AIDS (London, England) 2008;22(7):906–7. doi: 10.1097/QAD.0b013e3282f706ba. [DOI] [PubMed] [Google Scholar]
- 11.Fogel J, Li Q, Taha TE, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin Infect Dis. 2011;52(8):1069–76. doi: 10.1093/cid/cir008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn L, Hunt G, Technau KG, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS. 2014;28(11):1673–8. doi: 10.1097/QAD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung BH, Rezk NL, Bridges AS, Corbett AH, Kashuba AD. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2007;21(10):1095–104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 14.Derache A, Maiga AI, Traore O, et al. Evolution of genetic diversity and drug resistance mutations in HIV-1 among untreated patients from Mali between 2005 and 2006. J Antimicrob Chemother. 2008;62(3):456–63. doi: 10.1093/jac/dkn234. [DOI] [PubMed] [Google Scholar]
- 15.Maiga AI, Fofana DB, Maiga AC, et al. Transmitted antiretroviral drug resistance in newly HIV-infected and untreated patients in Segou and Bamako, Mali. AIDS Res Hum Retroviruses. 2013;29(1):182–6. doi: 10.1089/aid.2012.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loubser S, Balfe P, Sherman G, Hammer S, Kuhn L, Morris L. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. AIDS. 2006;20(7):995–1002. doi: 10.1097/01.aids.0000222071.60620.1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt GM, Coovadia A, Abrams EJ, et al. HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine. AIDS. 2011;25(12):1461–9. doi: 10.1097/QAD.0b013e3283492180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15(15):1951–7. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 19.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral concentrations in breastfeeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53(3):1170–6. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro RL, Holland DT, Capparelli E, et al. Antiretroviral concentrations in breastfeeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis. 2005;192(5):720–7. doi: 10.1086/432483. [DOI] [PubMed] [Google Scholar]
- 21.Schneider S, Peltier A, Gras A, et al. Efavirenz in human breast milk, mothers’, and newborns’ plasma. J Acquir Immune Defic Syndr. 2008;48(4):450–4. doi: 10.1097/QAI.0b013e31817bbc21. [DOI] [PubMed] [Google Scholar]
- 22.Palombi L, Pirillo MF, Andreotti M, et al. Antiretroviral prophylaxis for breastfeeding transmission in Malawi: drug concentrations, virological efficacy and safety. Antivir Ther. 2012;17(8):1511–9. doi: 10.3851/IMP2315. [DOI] [PubMed] [Google Scholar]
- 23.Palombi L, Pirillo MF, Marchei E, et al. Concentrations of tenofovir, lamivudine and efavirenz in mothers and children enrolled under the Option B-Plus approach in Malawi. J Antimicrob Chemother. 2015 doi: 10.1093/jac/dkv435. [DOI] [PubMed] [Google Scholar]
- 24.Corbett AH, Kayira D, White NR, et al. Antiretroviral pharmacokinetics in mothers and breastfeeding infants from 6 to 24 weeks post-partum: results of the BAN Study. Antivir Ther. 2014;19(6):587–95. doi: 10.3851/IMP2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahle EM, Kashuba A, Baeten JM, et al. Unreported antiretroviral use by HIV-1-infected participants enrolling in a prospective research study. J Acquir Immune Defic Syndr. 2014;65(2):e90–4. doi: 10.1097/QAI.0b013e3182a2db02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troyer RM, Lalonde MS, Fraundorf E, et al. A radiolabeled oligonucleotide ligation assay demonstrates the high frequency of nevirapine resistance mutations in HIV type 1 quasispecies of NVP-treated and untreated mother-infant pairs from Uganda. AIDS Res Hum Retroviruses. 2008;24(2):235–50. doi: 10.1089/aid.2007.0138. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JA, Li JF, Morris L, et al. Emergence of drug-resistant HIV-1 after intrapartum administration of single-dose nevirapine is substantially underestimated. J Infect Dis. 2005;192(1):16–23. doi: 10.1086/430741. [DOI] [PubMed] [Google Scholar]
- 28.Micek MA, Blanco AJ, Beck IA, et al. Nevirapine resistance by timing of HIV type 1 infection in infants treated with single-dose nevirapine. Clin Infect Dis. 2010;50(10):1405–14. doi: 10.1086/652151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jittamala P, Puthanakit T, Chaiinseeard S, Sirisanthana V. Predictors of virologic failure and genotypic resistance mutation patterns in thai children receiving non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy. Pediatr Infect Dis J. 2009;28(9):826–30. doi: 10.1097/INF.0b013e3181a458f9. [DOI] [PubMed] [Google Scholar]
- 30.Ruel TD, Kamya MR, Li P, et al. Early virologic failure and the development of antiretroviral drug resistance mutations in HIV-infected Ugandan children. J Acquir Immune Defic Syndr. 2011;56(1):44–50. doi: 10.1097/QAI.0b013e3181fbcbf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towler WI, Barlow-Mosha L, Church JD, et al. Analysis of drug resistance in children receiving antiretroviral therapy for treatment of HIV-1 infection in Uganda. AIDS Res Hum Retroviruses. 2010;26(5):563–8. doi: 10.1089/aid.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving antiretroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. Pediatr Infect Dis J. 2011;30(1):52–6. doi: 10.1097/INF.0b013e3181ed2af3. [DOI] [PubMed] [Google Scholar]
- 33.Mghamba FW, Minzi OM, Massawe A, Sasi P. Adherence to antiretroviral therapy among HIV infected children measured by caretaker report, medication return, and drug level in Dar Es Salaam, Tanzania. BMC Pediatr. 2013;13:95. doi: 10.1186/1471-2431-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]