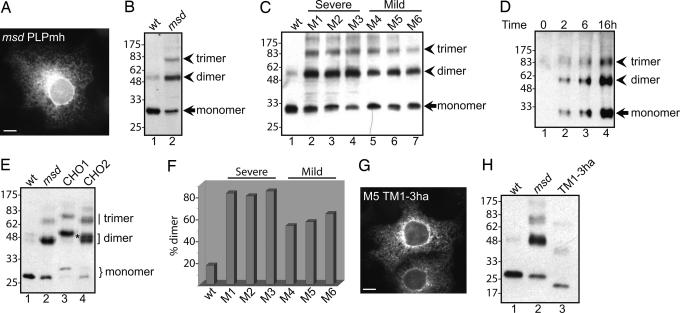
Fig. 3.
Oligomerization of PLP mutants. (A) COS-7 cells transiently transfected with msd PLPmh were stained with α-myc followed by a FITC-conjugated secondary antibody. (Scale bar: 20 μm.) (B) Lysates of COS-7 cells transfected with msd PLPmh were analyzed by SDS/PAGE and Western blotting with α-myc. (C) COS-7 cells were transfected with WT or mutant forms of PLPmh and then analyzed as in B. (D) A stable cell line was induced to express msd PLPmh for the time indicated and then analyzed as in B. Note that approximately three times more cell lysate was used here than in Fig. 2C. (E) Lysates of COS-7 cells transfected with WT or msd PLPha, or with msd PLPha containing a glycosylation site in the first (msd CHO1) or second (msd CHO2) luminal loop, were analyzed by SDS/PAGE and Western blotting with α-ha. The position of glycosylated and nonglycosylated monomeric PLPha is shown by a curly bracket, and dimers are indicated by a square bracket. A dimer composed of glycosylated and nonglycosylated PLP is shown by an asterisk. (F) COS-7 cells were transfected as in C and then analyzed by SDS/PAGE and Western blotting with α-myc followed by [125I]protein A. PLPmh was quantified by autoradiography, and the amount of SDS-resistant dimer is expressed as a percentage of the total amount of dimer plus monomer. The chart shows the means of two to three independent experiments. The significance of the difference between mutants associated with mild and severe disease was determined by using the independent-samples t test. (G) COS-7 cells were transfected with TM1-3ha and stained with α-ha followed by a FITC-conjugated secondary antibody. (Scale bar: 20 μm.) (H) COS-7 cells were transfected with WT or msd PLPha, or with mutant 5 TM1-3ha and analyzed as in E.