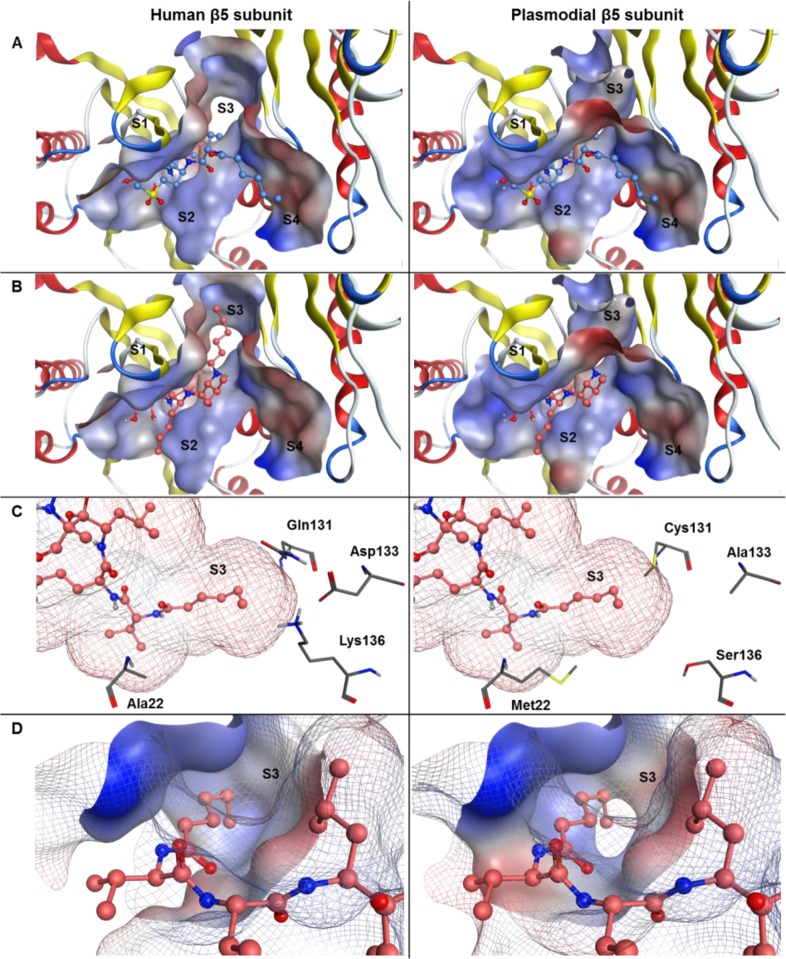
Figure 4.
Schematic representation of carmaphycin B (blue) and analog 18 (orange) in ball and stick representation bound to the human 20S proteasome β5 binding pocket (left, PDB code 4R67) and the P. falciparum 20S proteasome β5 binding pocket of the homology model homPf_β5 (right). The molecular surface of the protein binding pocket is shown with hydrophilic (blue) and hydrophobic (red) surface areas. (A) Carmaphycin B bound to the β5 subunit. (B) Analog 18 bound in the switched conformation. (C) Analog 18 binding conformation in the S3 protein pocket. The residues that were identified to be associated with the preferred binding of analog 18 toward the Plasmodium 20S proteasome β5 subunit are shown in gray. The interaction surface of the inhibitor analog 18 is color coded according to the lipophilicity of the molecule with hydrophilic (blue) and hydrophobic (red) areas. (D) Analog 18 binding conformation in the S3 protein pocket. The molecular surface of the S3 binding pocket is shown as solid surface areas, whereas the whole binding pocket is shown as line representation.