Scheme 1.
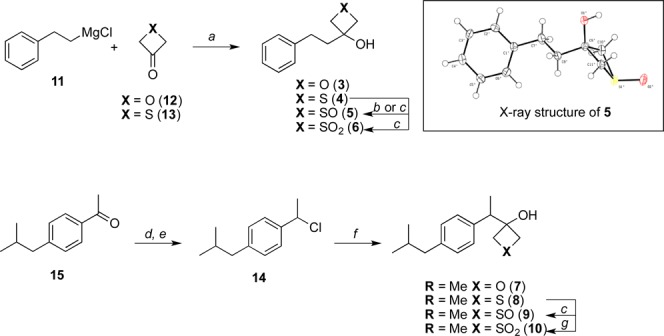
Reagents and reaction conditions: (a) THF, −78 °C, 15 min, then rt, 1 h; (b) UHP, AcOH, rt, 14 h; (c) m-CPBA, CH2Cl2, −78 °C, 1 h; (d) NaBH4, MeOH, 0 °C to rt, 30 min; (e) SOCl2, CH2Cl2, 0 °C to rt, 18 h; (f) (i) Mg, LiCl, ZnCl2, THF, rt, 6 h, (ii) 12 or 13, THF, rt, 17 h; (g) oxone, acetone/H2O, 0 °C to rt, 20 h.
