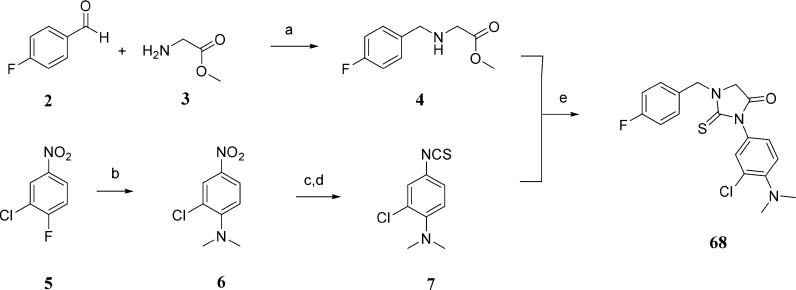
Scheme 1. Representative Synthesis of 2-Thiohydantoin Derivative 68.
Reagents and conditions: (a) Et3N, mol. sieves 3 Å, NaCNBH3, CHCl3, rt, yield 48%; (b) dimethylamine HCl salt, K2CO3, DMSO, 80 °C, yield 95%; (c) SnCl2, EtOAc/EtOH, 80 °C, yield 64%; (d) CSCl2, DCM-aq·NaHCO3, 4 °C–rt, yield 84%; (e) EtOH, rt, yield 70%.