Abstract
Background
Malignant pleural mesothelioma (MPM) is a rare and aggressive disease that poses a treatment challenge in spite of recent technical developments. The aim of this retrospective analysis is to assess the feasibility of administering intensity-modulated radiotherapy (IMRT) to the pleural cavity using helical tomotherapy in patients who had undergone pleurectomy/decortication (P/D) and also the resulting toxicity levels.
Patients and methods
Ten patients who had MPM and had undergone P/D were treated with pleural cavity irradiation that included a median dose of 52.2 Gy using helical tomotherapy. The median age of the patients was 53 years (31–74). In addition to clinical and diagnostic findings from regular follow-up examinations, we evaluated the dose distribution for other organs at risk to assess treatment in relation to toxicity, with special regard for the underlying intact lung.
Results
The mean lung dose on the treatment site was 32.8 Gy (±6.8). The V20 Gy was 71.7% (±17.2). No treatment-related toxicity that exceeded grade III according to common toxicity criteria (CTC) was observed. Median progression-free survival (PFS) was 13 months with a median overall survival (OAS) of 19 months.
Conclusion
The findings of this analysis provide data indicating that sparing the underlying lung in patients with MPM after P/D is not only feasible with helical tomotherapy, but that this treatment also causes reasonably few side effects.
Keywords: Malignant pleural mesothelioma, Pleural cavity irradiation, Pleurectomy/decortication, Tomotherapy
1. Background
Malignant pleural mesothelioma (MPM) is a rare disease, and its treatment still poses a clinical challenge. Because of its biological aggressiveness and propensity for local and distant propagation, multimodal treatment approaches are necessary.1, 2 There are two surgical procedures available for patients with MPM. Extrapleural pneumectomy (EPP) involves en bloc resection of the involved lung, parts of the diaphragm, and the parietal pleura and pericardium, while pleurectomy/decortication (P/D) is an alternative surgical option in which the underlying lung can be spared and left in situ.3 EPP, as the more radical approach,4 has been used less frequently since comparisons of the two approaches showed a clear advantage with P/D in terms of morbidity and perioperative mortality.5 Data from the recently published Mesothelioma and Radical Surgery (MARS) Trial substantiate these findings and have even led to the conclusion that EPP, as part of the trimodality treatment, is not only not beneficial but potentially harmful for patients.6 Therefore, it appears that the choice of surgery that is performed may affect the patient's eligibility for further adjuvant therapy. The increase in the number of P/D treatments performed is of particular importance in terms of the treatment techniques for adjuvant radiotherapy. On the one hand, because there is less extensive resection, the risk for local recurrence might be aggravated and, therefore, no compromises should be made on the dose prescription. However, on the other hand, organ preservation must be taken into account through an improved ability to spare organs at risk (OAR). In general, intensity modulated radiotherapy (IMRT) seems to be a suitable technique for addressing this challenge7, 8, 9 and, as we have already shown, helical tomotherapy in particular allows excellent and homogenous target coverage in patients after EPP.10 However, the requirements for treating only the pleural cavity while sparing the intact lung as much as possible pose a much greater challenge in terms of both equipment and medical personnel. Recent studies have shown the feasibility and the acceptable toxicity profiles for adjuvant IMRT.11 While plenty of validated constraints are available for the treatment of central lung tumors, generally accepted constraints for particular dose distribution of pleural cavity irradiation (high doses around the lung) are still missing. This retrospective work investigates the feasibility and clinical outcomes of adjuvant radiotherapy after P/D using helical tomotherapy, with special regard to the dosimetric parameters in terms of the ability to spare OARs.
2. Patients and methods
2.1. Patients’ characteristics
Between September 2007 and March 2013, ten patients (seven males; three females) with histological proven MPM (six right-sided; four left sided) were treated adjuvantly after P/D. The median age of the patients was 53 years (31–74 years). All patients received four to six cycles of chemotherapy (a combination of cisplatin and pemetrexed) either neoadjuvantly (n = 2) or postoperatively before the initiation of radiotherapy (n = 8). Additional patient characteristics are listed in Table 1.
Table 1.
Patient characteristics.
| Patient characteristics (n = 10) | |
|---|---|
| Age | 53 years (31–74) |
| Gender | |
| Male | 7 |
| Female | 3 |
| Laterality | |
| Right | 6 |
| Left | 4 |
| Histology | |
| Epitheloid | 6 |
| Sarcomatoid | 2 |
| Biphasic | 2 |
| Stage | |
| I | 3 |
| II | 1 |
| III | 4 |
| IV | 2 |
| Gross residual disease | |
| Yes | 4 |
| No | 6 |
| Previous treatment with chemotherapy | |
| Yes | 10 |
| No | 0 |
2.2. Treatment planning and radiotherapy
IMRT was conducted as helical tomotherapy with 6 MeV photons. The treatment beam was conformed using a binary multileaf collimator. A median dose of 52.2 Gy (40–54 Gy) was applied in conventional fractionation of 2 Gy single doses five times a week with a median treatment time of 839.8 s (478–1281.4 s). Four patients received an integrated boost to macroscopic residual disease up to 60 Gy. Boost volume was defined as macroscopic tumor as seen on contrast enhanced CT scan expanded by 5 mm resulting in a mean boost volume of 337 cc. Additional boost irradiation had no significant impact on dose distribution for OARs. Patients were immobilized in the supine position with their arms over their heads and using chest boards. For inverse treatment planning, Accuray's tomotherapy treatment planning station was used with a field width of 2.5 cm. The contour of the pleural cavity was isotropically expanded from the apex of the lung to the costophrenic recess by 5 mm; a margin of 1 cm was used inferior of the diaphragm to account for breathing motion. No additional breathing motion management was applied. Mean pleural cavity volume was 2649 cc. Interlobar space was not contoured, to minimize the dose to the lung since it had not been affected in any of the patients.
2.3. Statistics
Overall survival (OAS) and progression-free survival (PFS) were calculated as being from the beginning of radiotherapy until the time of death or the last documented follow-up visit (Kaplan–Meier-estimator, Sigma Plot 12.5, Systat Software). In addition to clinical and diagnostic findings from regular follow-up examinations (every 3 months), we evaluated the dose distribution of OARs to assess treatment-related toxicity, with special regard to the underlying intact lung. Details on dose distribution for OARs in relation to the treatment side can be seen in Table 2. Analysis of tests for pulmonary function before and after radiotherapy was visualized using Sigma Plot 12.5, Systat Software.
Table 2.
Analysis of mean OAR dose ± standard deviation.
| Dose distribution of OAR | |||||
|---|---|---|---|---|---|
| PTV | Contralateral lung | ||||
| Total dose | 52.2 Gy | ±3.6 | Average | 8.3 Gy | ±1.7 |
| Dose per fraction | 2 Gy | ±0.1 | V20 Gy | 2.2% | ±2.5 |
| Treatment duration | 839.8 s | ±259.9 | |||
| Lung (treatment side) | |||||
| Average | 32.8 Gy | ±6.8 | |||
| D10% | 51.1 Gy | ±6.9 | V10 Gy | 95.9% | ±8.1 |
| D20% | 47.1 Gy | ±7.2 | V20 Gy | 71.7% | ±17.2 |
| D30% | 42.8 Gy | ±7.4 | V30 Gy | 54.6% | ±18.8 |
| D40% | 37.6 Gy | ±8.3 | V40 Gy | 37.7% | ±19.1 |
| D50% | 32.3 Gy | ±9.7 | V50 Gy | 17.9% | ±15.3 |
| Heart (for right-sided RT) | |||||
| Average | 22.9 Gy | ±4.9 | |||
| D20% | 33.0 Gy | ±8.8 | V20 Gy | 46.0% | ±14.9 |
| D40% | 22.1 Gy | ±5.4 | V40 Gy | 13.3% | ±8.6 |
| Heart (for left-sided RT) | |||||
| Average | 26.4 Gy | ±3.1 | |||
| D20% | 36.9 Gy | ±3.4 | V20 Gy | 61.3% | ±15.4 |
| D40% | 26.9 Gy | ±5.8 | V40 Gy | 17.4% | ±4.5 |
| Esophagus | Spinal cord | ||||
| Average | 27.0 Gy | ±13.2 | Dmax | 34.5 Gy | ±5.5 |
| D40% | 28.5 Gy | ±15.1 | |||
| Dmax | 50.4 Gy | ±7.8 | |||
| V40 Gy | 23.7% | ±31.1 | |||
| Liver (for right-sided RT) | Liver (for left-sided RT) | ||||
| Average | 25.3 Gy | ±5.2 | Average | 10.4 Gy | ±4.0 |
| Kidney (treatment side) | Contralateral kidney | ||||
| Average | 7.6 Gy | ±3.3 | Average | 4.0 Gy | ±1.6 |
2.4. Ethical approval
The collection and analysis of the data was performed in accordance with the Declaration of Helsinki and the guidelines of the institution's ethics committee.
3. Results
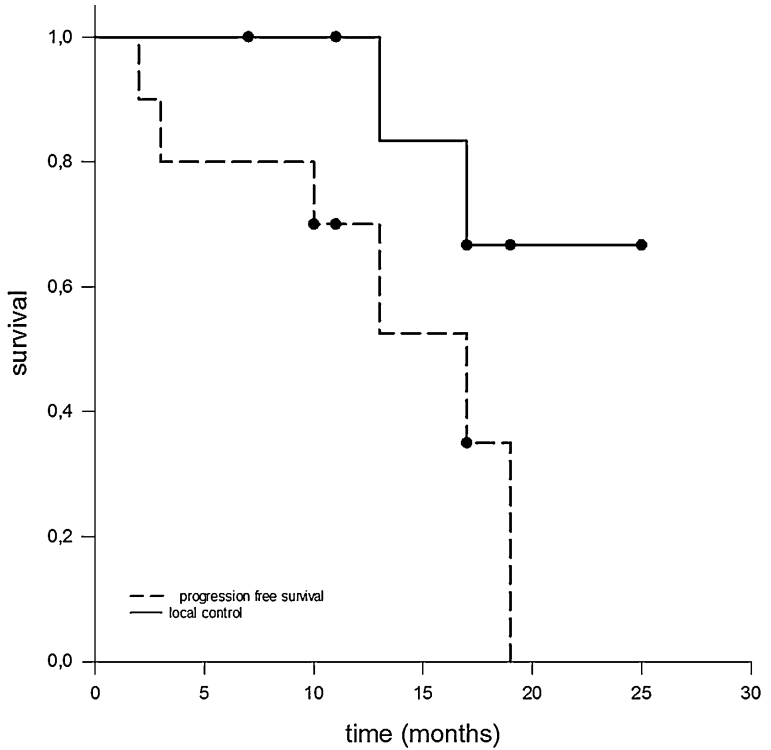
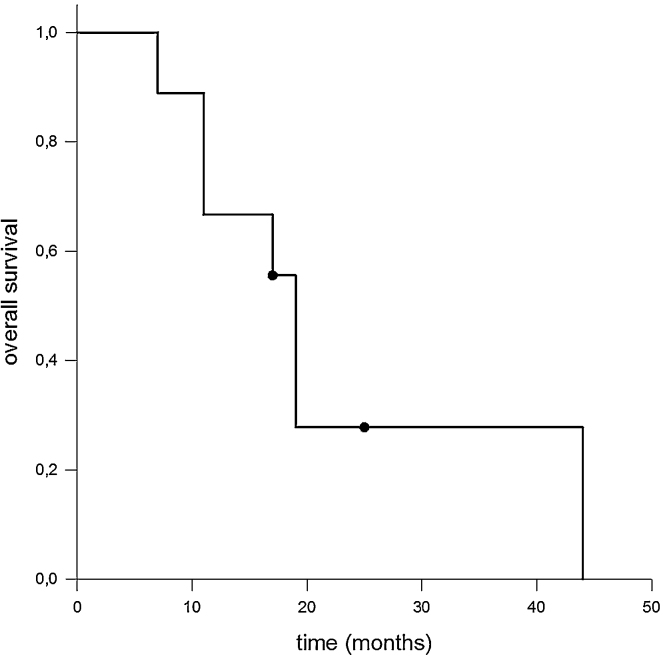
All patients were able to complete the radiotherapy as prescribed. No acute treatment-related toxicity exceeding grade I according to common terminology criteria for adverse events CTCAE was observed. Median follow-up time from the beginning of treatment was 17 months. During this period, patients were seen quarterly either by us or by a partner institution and underwent clinical and radiological follow-ups. Fig. 1, Fig. 2 show Kaplan–Meier estimators for PFS and OAS. Median PFS was 13 months; however, it should be noted that in only two cases was there local failure in terms of in-field recurrence; one case with integrated boost concept and one without. Heterogeneous distant progression was detected in four patients (contralateral diaphragm, pulmonary, osseous, cerebral, and intra-abdominal). Of these patients, two were treated with an integrated boost concept. Other two patients died for reasons that were not directly related to the underlying disease. The remaining two patients are in good general condition, most recently showing a stable oncologic follow-up.
Fig. 1.
Kaplan–Meier estimates for PFS and for local control. Local failure in terms of in-field recurrence was only observed in two patients, resulting in a satisfying local control rate. Although propensity for propagation in MPM is pronounced, the median PFS was 13 months.
Fig. 2.
Kaplan–Meier estimates for OAS. In spite of the aggressiveness of and unfavorable prognosis for MPM, the median OAS observed in our cohort was 19 months. However, the impact of modern radiation techniques on OAS has still to be clarified.
During follow-up, two patients developed radiogenic pneumonitis. Three months after completion of the treatment, a 63-year-old female patient reported progressive dyspnea with sputum and distinct feelings of illness. The clinical suspicion was able to be confirmed radiologically, and radiogenic pneumonitis II according to CTCAE was diagnosed. Following the appropriate application of corticosteroids her symptoms showed remission. The second case was more severe. A 48-year-old woman required hospitalization six weeks after completing the therapy because of radiogenic pneumonitis III with a transient necessity for oxygen application. Fortunately, the patient recovered quickly after an appropriate therapy was initiated.
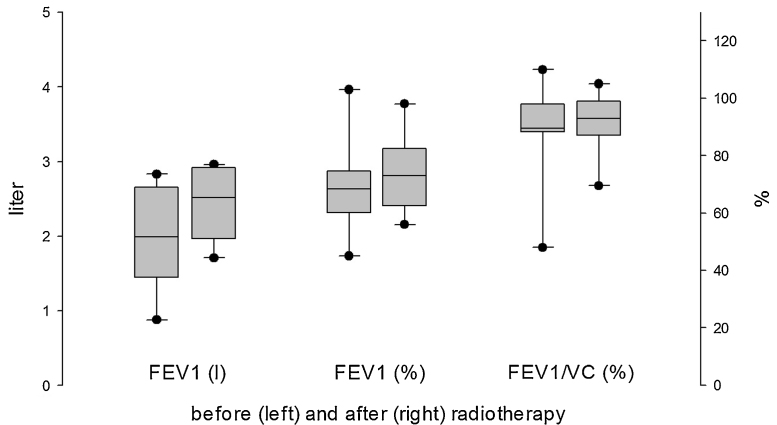
Testing for pulmonary function was performed before and after radiotherapy. With respect to forced expiratory volume per second, a slight improvement was observed during follow-up examinations, although this proved not to be significant (Fig. 3).
Fig. 3.
Analysis of pulmonary function before (left) and after (right) conducting radiotherapy resulted in no significant changes. However, the tests showed a tendency toward slightly enhanced forced expiratory volume per second.
Treatment plans for risk stratification were retrospectively analyzed, and the average dose for OARs and several other relevant dose points were determined. Table 2 shows details about the dose distribution of the OARs, according to the treatment sites. The review of the data clearly shows the high dose received by the remaining intact lung: the mean lung dose (MLD) was 32.8 Gy (21.5–47.2 Gy) with an average V20 Gy = 71.7% (45–100%), while the contralateral lung was able to be spared (MLD 8.3 Gy, V20 Gy 2.3%). As expected, significant differences could be seen for the heart, liver, and kidneys, depending on the laterality of the target volume (Table 2).
4. Discussion
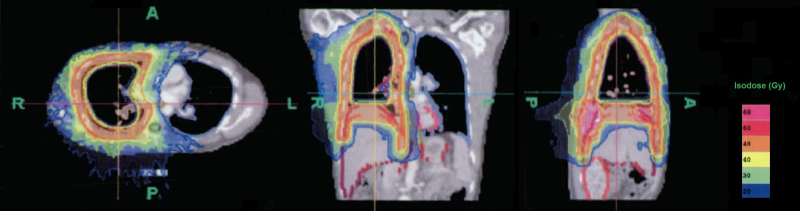
The risk for radiogenic pneumonitis in relation to the MLD can be estimated by using the existing data for the normal tissue tolerance of the quantitative analysis of normal tissue effects in the clinic (QUANTEC).12 Based on this, our recommendation is to limit V20 Gy to a value of less than 30–35% and the MLD to 20–23 Gy. The risk for higher-grade radiogenic pneumonitis does not exceed 20% when adhering to the above-mentioned dose constraints and using conventional fractionation. Applying this risk assessment to our patient group, a complication rate of >50% is expected for an MLD > 32 Gy. In fact, treatment-related lung toxicity occurred in only two of the 10 cases. These two patients did not differ from the rest of the group in terms of their previous chemotherapy, nor were their dose distributions significantly higher, especially since they had no macroscopic residual tumor and, hence, did not receive a boost irradiation. Neither the MLD (33.9 Gy and 33.2 Gy) nor the V20 Gy (80% and 62.5%) did differ significantly from the collective mean values (32.8 Gy and 71.7%). With respect to the fact that QUANTEC data is based mainly on 3D-conformal techniques for lung cancer, it is important that discussion takes place in the future regarding how those findings can be transferred without hesitation. However, the extraordinarily high doses received by the underlying intact lung remain indisputable. Clearly, the relationship between the dose and the volume is not the single determining factor for the occurrence of therapy-related toxicity. It is possible that the role played by dose distribution is greater than was previously assumed. Fig. 4 shows that, as determined by the anatomy, the high-dose area concentrates predominantly on the outer perimeter of the lung and declines rapidly as a result of the steep dose gradient that is achieved. In accordance with Timmerman et al.,13 who observed an excessive rise in toxicity in treatment of central lung tumors, defined as a 2 cm perimeter around the bronchial tree, the risk for radiogenic pneumonitis could be overestimated because of the predominantly peripheral dose distribution. Because MPM is so rare, reports on it are limited. The analysis with the largest patient group (n = 67) originates from Rimner et al.14 This provides detailed information about the patterns of failure among patients who had two intact lungs and received hemithoracal pleural IMRT after P/D. The analysis concludes with the findings of Rosenzweig et al. that local recurrence prevails.15 In this series of 36 patients, one lethal case of radiogenic pneumonitis was observed in the post-therapeutic course, in addition to six other cases with grade III or IV (median dose applied: 46.8 Gy, range 41.4–50.4 Gy). The observed complication rate of 20% in this study is, then, comparable to the previous reports. Minatel et al. also published a report showing that a quarter of 20 patients who were treated were diagnosed with pneumonitis (prescription dose: 50 Gy in 25 fractions).16
Fig. 4.
Exemplary dose distribution for pleural cavity irradiation using helical tomotherapy showed a steep dose gradient for sparing OARs, especially from the outer perimeter of the lung toward more central parts of the remaining lung.
A report by Rice et al. indicates that sparing the contralateral lung is of particular importance in patients with EPP.17 Death related to lung toxicity strongly correlates with exposed volume. This report shows increasing risk with MLD > 8.5 Gy, V5 Gy > 75%, or V20 Gy > 7%. Krayenbuehl et al. have recommended trying to keep the MLD below 10 Gy with V20 Gy < 10% and V5 Gy < 50%.18 Also, although the feasibility and dosimetric evaluation of helical tomotherapy has previously been published,19 these studies focused on patients who received EPP rather than P/D, which is why, for obvious reasons, lung constraints have to be very strict. The dosimetric values achieved in this study (MLD for the contralateral lung was 8.3 Gy with a V20 Gy of 2.2%) accentuate the potential for sparing OARs.
The low rate of 20% for local recurrence seems to be discordant with other reports. No dependency could be found, whether an integrated boost was applied or not. An increasing local control can be explained, to a certain degree, by the technical progress and increasing clinical experience concerning the target-volume definition that Rimner et al. reported and which was discussed earlier.14 However, the relatively small number of cases must be taken into account as possibly leading to a certain distortion in the results. Therefore, we evaluated the pulmonary function before and after radiotherapy to consolidate our findings with additional clinical data. The fact that no significant change in the relevant pulmonary parameters was observed (especially no deterioration), and that survival data showed improvement (with a median OAS of 19 months) allows a cautious optimism that, with adjuvant irradiation of the pleural cavity, another effective therapeutic option is available with a potential that has yet to be reached. Further technical advances as well as standardization of treatment concepts within larger prospective settings are necessary to ensure that patients with this rare disease are offered the best therapy available.
5. Conclusion
Our results indicate that helical tomotherapy is a feasible therapeutic option for patients with MPM after P/D with a reasonable toxicity profile concerning the remaining intact lung, as long as the interlobar space is not affected.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Stahel R.A. Malignant pleural mesothelioma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl. 4):piv73–piv75. doi: 10.1093/annonc/mdp134. [DOI] [PubMed] [Google Scholar]
- 2.Moore A., Parker R., Wiggins J. Malignant mesothelioma. Orphanet J Rare Dis. 2008;3(1):34. doi: 10.1186/1750-1172-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores R.M. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg. 2009;21(2):149–153. doi: 10.1053/j.semtcvs.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker D.J., Garcia J.P. Multimodality therapy for malignant pleural mesothelioma. Chest. 1997;112(Suppl. 4):272S–275S. doi: 10.1378/chest.112.4_supplement.272s. [DOI] [PubMed] [Google Scholar]
- 5.Cao C. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer. 2014;83(2):240–245. doi: 10.1016/j.lungcan.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Treasure T. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12(8):763–772. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miles E.F. Intensity-modulated radiotherapy for resected mesothelioma: the Duke experience. Int J Radiat Oncol Biol Phys. 2008;71(4):1143–1150. doi: 10.1016/j.ijrobp.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Rimner A., Rosenzweig K.E. Novel radiation therapy approaches in malignant pleural mesothelioma. Ann Cardiothorac Surg. 2012;1(4):457–461. doi: 10.3978/j.issn.2225-319X.2012.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maciejczyk A., Skrzypczyńska I., Janiszewska M. Lung cancer. Radiotherapy in lung cancer: actual methods and future trends. Rep Pract Oncol Radiother. 2014;19(6):353–360. doi: 10.1016/j.rpor.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sterzing F. Evaluating target coverage and normal tissue sparing in the adjuvant radiotherapy of malignant pleural mesothelioma: helical tomotherapy compared with step-and-shoot IMRT. Radiother Oncol. 2008;86(2):251–257. doi: 10.1016/j.radonc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Minatel E.M.D. Tomotherapy after pleurectomy/decortication or biopsy for malignant pleural mesothelioma allows the delivery of high dose of radiation in patients with intact lung. J Thorac Oncol. 2012;7(12):1862–1866. doi: 10.1097/JTO.0b013e318272601f. [DOI] [PubMed] [Google Scholar]
- 12.Marks L.B. Radiation dose–volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timmerman R. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 14.Rimner A. Failure patterns after hemithoracic pleural intensity modulated radiation therapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2014;90(2):394–401. doi: 10.1016/j.ijrobp.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenzweig K.E. Pleural intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2012;83(4):1278–1283. doi: 10.1016/j.ijrobp.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minatel E. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer. 2014;83(1):78–82. doi: 10.1016/j.lungcan.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Rice D.C. Dose-dependent pulmonary toxicity after postoperative intensity-modulated radiotherapy for malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys. 2007;69(2):350–357. doi: 10.1016/j.ijrobp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Krayenbuehl J. Combined photon and electron three-dimensional conformal versus intensity-modulated radiotherapy with integrated boost for adjuvant treatment of malignant pleural mesothelioma after pleuropneumonectomy. Int J Radiat Oncol Biol Phys. 2007;69(5):1593–1599. doi: 10.1016/j.ijrobp.2007.07.2370. [DOI] [PubMed] [Google Scholar]
- 19.Giraud P. Helical tomotherapy for resected malignant pleural mesothelioma: dosimetric evaluation and toxicity. Radiother Oncol. 2011;101(2):303–306. doi: 10.1016/j.radonc.2011.06.040. [DOI] [PubMed] [Google Scholar]