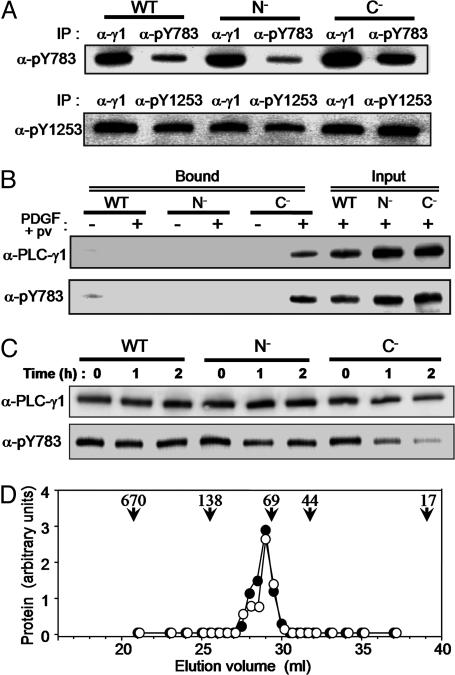
Fig. 3.
Intramolecular association of pY783 with the SH2(C) domain in PLC-γ1. (A) Null TV-1 cells expressing wild-type, N–,orC– forms of PLC-γ1 were stimulated for 10 min with PDGF plus pervanadate, after which the PLC-γ1 proteins were partially purified from cell lysates by batch chromatography on heparin-Sepharose. The partially purified proteins were then subjected to immunoprecipitation with anti-PLC-γ1 (α-γ1), -pY783, or -pY1253, and the resulting precipitates were subjected to immunoblot analysis with the same antibodies. (B) Partially purified PLC-γ1 proteins prepared as in A from non-stimulated or cells stimulated with PDGF plus pervanadate were incubated with a GST fusion protein containing the SH2(N)-SH2(C)-SH3 domains of PLC-γ1. Proteins that bound to the GST fusion protein were precipitated with GSH-Sepharose and subjected to immunoblot analysis with the indicated antibodies (Bound). The partially purified PLC-γ1 proteins equivalent to one-fourth of the amount used for the binding assay were also subjected directly to immunoblot analysis (Input). (C) Null TV-1 cells were stimulated as in A and then lysed in the absence of phosphatase inhibitors (NaF, vanadate). The lysates were incubated in the presence of 5 mM DTT and 5 mM MgCl2 to activate phosphatases at 30°C for the indicated times and then subjected to immunoblot analysis with the indicated antibodies. (D) Null TV-1 cells expressing wild-type PLC-γ1 were left unstimulated or stimulated for 10 min with PDGF plus pervanadate. PLC-γ1 was then partially purified from cell lysates as in A and subjected to gel filtration by HPLC. The fractions from unstimulated (filled circles) and stimulated (open circles) cells were subjected to immunoblot analysis with anti-PLC-γ1 and anti-pY783, respectively. The elution positions of marker proteins are also shown: 670 kDa, thyroglobulin; 138 kDa, BSA dimer; 69 kDa, BSA monomer; 44 kDa, ovalbumin; and 17 kDa, myoglobin.