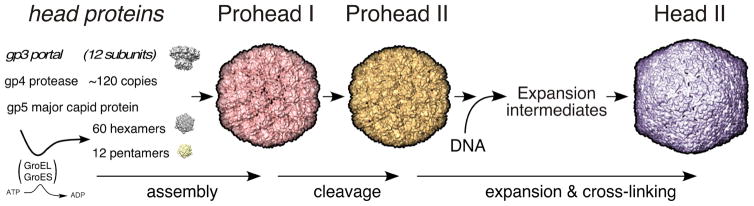
Figure 1. The HK97 capsid assembly pathway.
The HK97 major capsid protein (gp5, also abbreviated “mcp”) folds with the aid of GroEL and GroES and assembles into pentamers and hexamers. These co-assemble with the protease (gp4) and the portal protein (gp3, if present) to form Prohead I. After assembly, the protease removes the N-terminal 102-residue delta domain of gp5 creating the procapsid, Prohead II. The delta domains act like the scaffolding proteins of other phages and Herpesviruses. DNA packaging normally induces maturation to Head II. Maturation includes an expansion in size and conformational changes in all subunits. Stabilization of Head II is aided by the formation of covalent crosslinks between gp5* subunits during expansion.