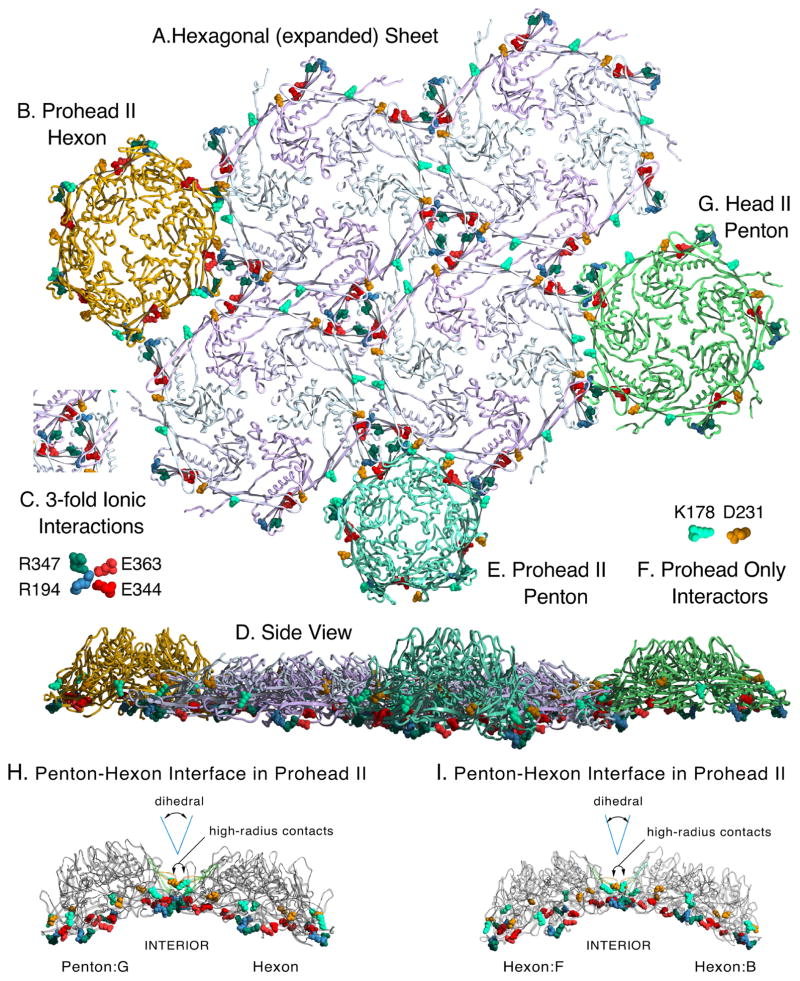
Figure 8. Modeled sheet of expanded and crosslinked mutant HK97 hexons and docking of related structures.
A. A model of a hexagonal sheet of HK97 major capsid protein made by rigid-body manipulations of six copies of chain A from the HK97 Head II (PDB ID: 1OHG). Several important charged contact residues at the interfaces of adjacent capsomers are shown in space-filling mode, color coded as indicated in insets C. and F. Those in C. are known to be preserved in all HK97 capsid structures, from prohead to head. We also believe that all of the highlighted residues provide electrostatic steering to align capsomer interfaces during assembly. We highlight the same residues in the following panels, which show docking of capsomers (from Prohead II and Head II atomic models) to the sheet model. The crosslink residues N356, K169 are in a position to form covalent bonds between hexon sheet subunits, but are hidden. B. Docking of a Prohead II hexon (chains A–F from PDBID: 3E8K), showing a poor fit for binding. C. Key to four residues involved in inter-capsomer ionic interactions are shown with color-coding as labeled. D. A side view of the entire array of capsomers shown in panels A. B. E. and G. E. Docking of a Prohead II penton (5 chains G from PDBID: 3E8K) F. Key to coloring of residues K178 and D231 which form a salt bridge in proheads, but not in heads. G. Docking of a Head II penton (5 chains G from PDBID: 1OHG). (protruding N-arms of Head II subunits were truncated when they would interfere or obscure the dockings). H. Side views of the contacts between a penton and a hexon in Prohead II. D231 and K178, which determine the dihedral angle between subunits on adjacent capsomers, are shown in space-filling mode to show that they are on the “hillsides” above the main contacts. P-domain ionic contact residues are also shown in space-filling mode. I. Same as (H) except contacts between two hexons are shown.