Figure 1.
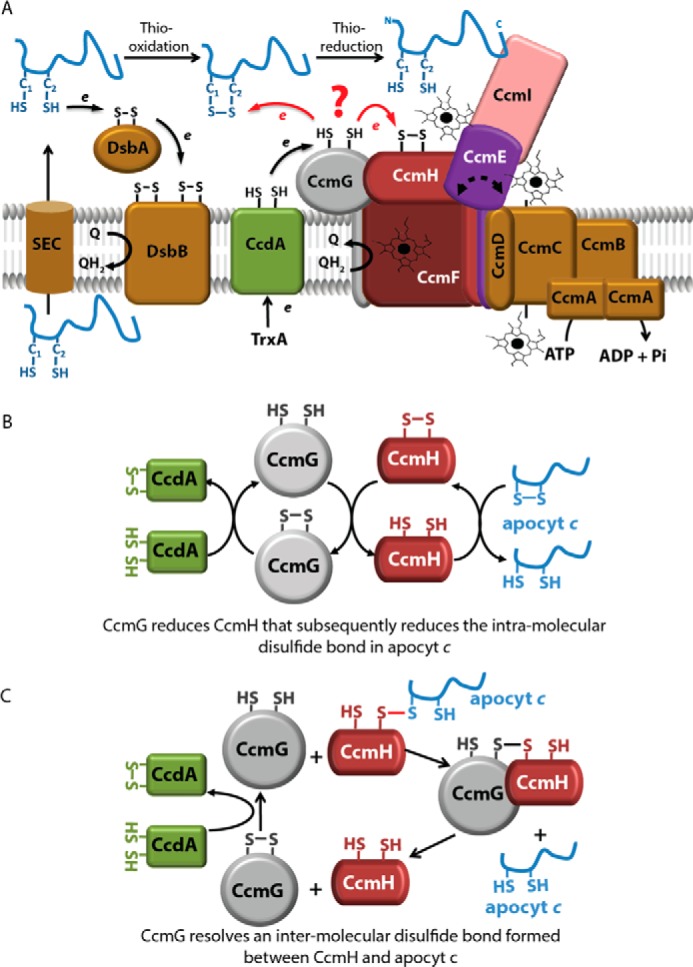
R. capsulatus Ccm system I and earlier proposed models for thioreduction of apocyt c HBS disulfide. A, nine membrane integral proteins (CcmABCDEFGHI) with different functions are responsible for covalent heme ligation to the apocyts to produce mature c-type cyts. Apocyts c are translocated via the SEC system to the periplasm, where the Cys residues at their HBS are oxidized by the DsbA–DsbB system (thio-oxidation). CcdA receives electrons from the cytoplasmic thioredoxin TrxA and reduces CcmG. The thiol-disulfide oxidoreductases CcmG and CcmH reduce the intramolecular disulfide bond at the apocyt HBS to allow heme ligation (thioreduction). CcmH together with CcmF and CcmI form the heme ligation core, whereas CcmABCD is an ATP-dependent ABC-type transporter that loads heme to CcmE to produce holo-CcmE. CcmI traps the C termini of the apocyt c substrates, whereas the heme delivered by holo-CcmE is covalently ligated to the apocyts c heme-binding sites by the CcmFHI core complex. B, CcmG–CcmH–apocyt c linear cascade of thiol-disulfide exchange. This proposal suggests that reduced CcmH (recycled by CcmG and CcdA) reduces directly apocyt c HBS disulfide bond (13, 24–27). C, CcmG reduces a mixed disulfide between CcmH and apocyt c. This proposal suggests that CcmG reduces a mixed disulfide formed between CcmH and apocyt c instead of oxidized CcmH or apocyt c HBS disulfide (12, 19, 25, 27).
