Figure 7.
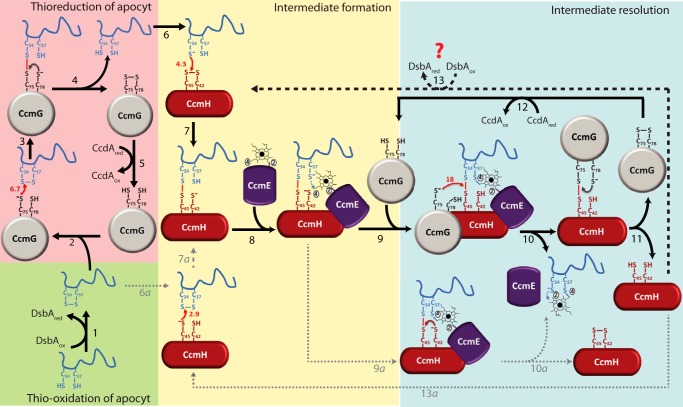
Thioreduction of the disulfide bond at the HBS of apocyt c and stereo-specific heme ligation during Ccm. The comprehensive model depicted here uses apocyt c1 as an example, but the process is considered to be similar with other apocyts c as well. In wild-type cells, after translocation to the periplasm, apocyt c1 is oxidized by the thiol-disulfide oxidoreductase DsbA, resulting in the formation of a disulfide bond at its HBS (34CXXCH38) (step 1). Ccm-specific thioredoxin CcmG carries a nucleophilic attack via its N-terminal, solvent exposed Cys-75 to the N-terminal Cys-34 at the HBS of apocyt c1 (steps 2 and 3), forming CcmGCys-75–Cys-34apocyt c1 mixed disulfide bond between them (step 3). The C-terminal Cys-78 of CcmG resolves this mixed disulfide bond, resulting in reduced apocyt c1 and oxidized CcmG (step 4), which is then re-reduced by CcdA (via electrons coming from cytoplasmic thioredoxins, not shown) (step 5). Reduced apocyt c1 attacks oxidized CcmH with its N-terminal Cys-34 (step 6), forming an intermediate in which apocyt c1 Cys-34 forms a mixed disulfide with the C-terminal highly reactive Cys-45 of CcmH (step 7), defining the stereo-specificity of heme ligation. Once the CcmHCys-45–Cys-34apocyt c1 intermediate is formed, holoCcmE carrying heme attached via its vinyl-2 is assumed to interact with CcmH and apocyt c1 (step 8), leading to the formation of the first thioether bond between the available Cys-37 of apocyt c1 and vinyl-4 of heme. The CcmH–apocyt c1–heme–CcmE intermediate thus formed is resolved efficiently via Cys-75 of CcmG, attacking this mixed disulfide (step 9), to form a mixed disulfide with Cys-45 of CcmH and releasing apocyt c1 (step 10). The Cys-34 of apocyt c1 is then assumed to form the second thioether bond with vinyl-2 of heme and released from CcmE (possibly involving CcmF, not shown). The CcmGCys-75–Cys-45CcmH mixed disulfide bond is resolved via Cys-78 of CcmG, rendering CcmH reduced and CcmG oxidized (step 11). As before (steps 2–4), CcdA recycles oxidized CcmG (step 12), and CcmH is oxidized via DsbA or another periplasmic oxidant(s) (step 13, heavy dashed line). Conceivably, the CcmHCys-45–Cys-34apocyt c1 intermediate may also be formed by an alternative route via a nucleophilic attack of the HBS disulfide bond by Cys-45 of reduced CcmH (steps 6a–7a), and be resolved via Cys-42 of CcmH, yielding oxidized CcmH (steps 9a–10a). However these steps (indicated by lightly dotted arrows) are less favorable as CcmH is mainly oxidized in vivo, and Cys-42 of CcmH is not very reactive. The thiol–disulfide exchange rates k (102 m−1 s−1, small red arrows) determined in this work are used to develop this scheme, where the resolution of intra-molecular disulfide bonds is indicated in small brown or gray arrows.