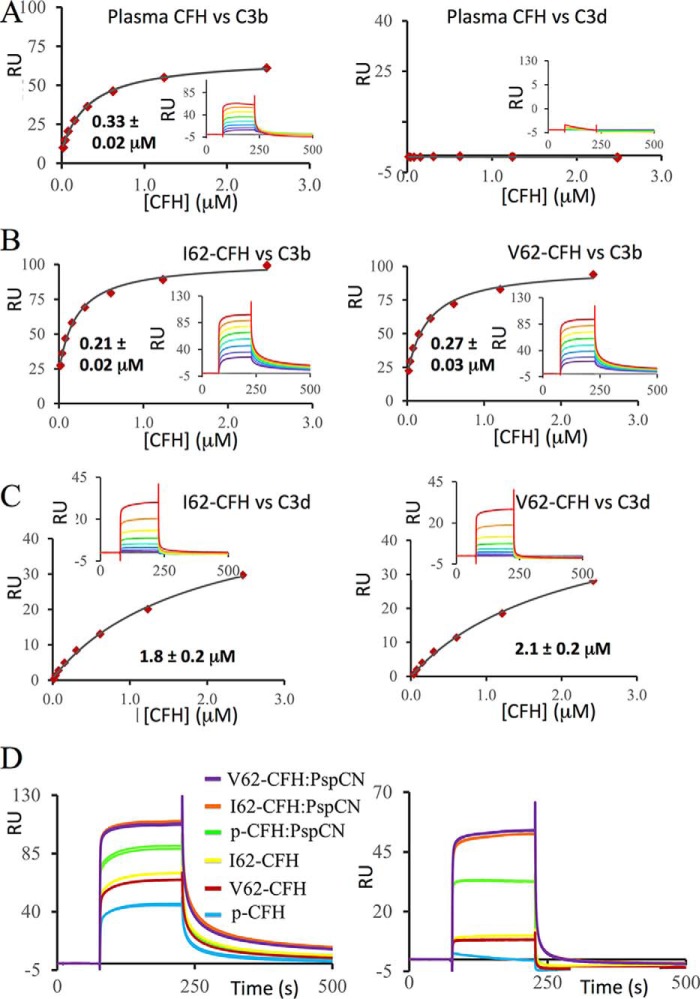
Figure 2.
C3b and C3d binding of plasma-purified CFH, I62-CFH, and V62-CFH. All data shown here were collected in one SPR experiment on the same C1-sensor chip. Care was taken to ensure that the CFH samples were all of equivalent quality and concentration. A, 2-fold dilution series (from 2.5 to 0.019 μm) of CFH from plasma were flowed in duplicate over amine-coupled C3b (left) or C3d (right). Equilibrium plots were used in an attempt to estimate KD values (Table 1), but no affinity measurement could be made for C3d (inset, background-subtracted sensorgrams). B, 2-fold dilution series (from 2.5 to 0.019 μm) of I62-CFH (left) or V62-CFH (right) were flowed in duplicate over amine-coupled C3b, and KD values were estimated as above. Two further experiments comparing V62-CFH with plasma CFH indicated similar binding to C3b. C, an equivalent set of experiments to B but with C3d instead of C3b. Multiple replicates confirmed the discrepancy in C3d binding between recombinant CFH and plasma CFH. D, sensorgrams for I62-CFH, V62-CFH, or plasma CFH (p-CFH) all at 0.6 μm, with or without PspCN (at 1.2 μm), flowed over C3b (left) or C3d (right). Very similar PspCN-induced enhancements of both plasma CFH and recombinant CFH binding to C3b and C3d were observed in multiple experiments; KD values were estimated for I62-CFH alone versus I62-CFH–PspCN complex (see Table 1).