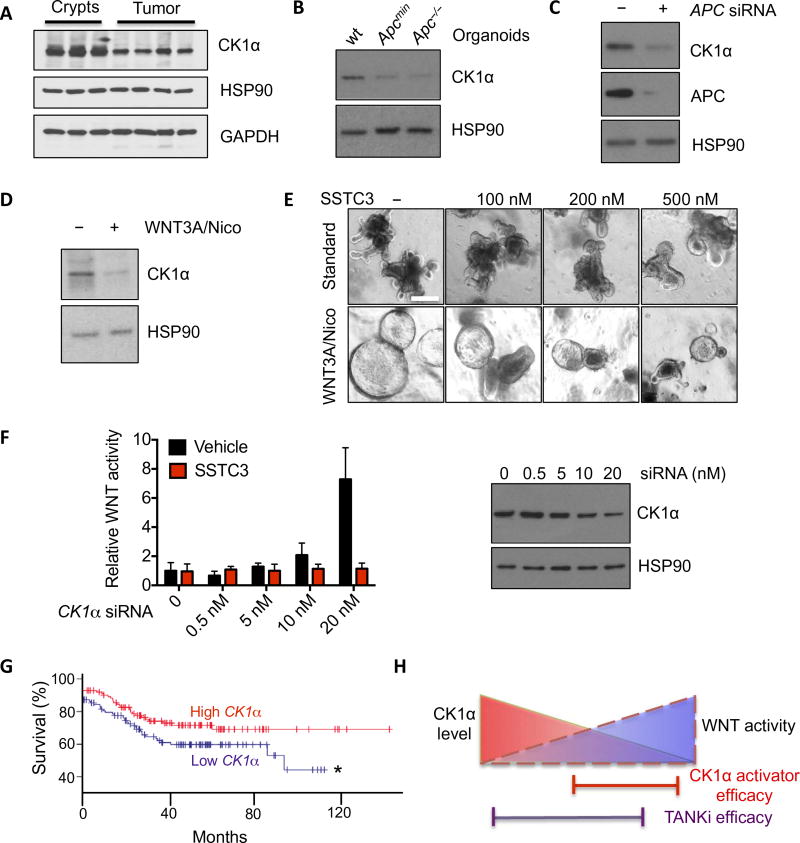
Fig. 6. CK1α abundance dictates SSTC3 sensitivity.
(A) Representative immunoblot for CK1α abundance in intestinal crypts or tumors isolated from wt or Apcmin mice. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Immunoblotting for CK1α abundance in organoids derived from wt mouse intestines or Apc mutant tumors. (C) Representative immunoblot for CK1α abundance in 293T cells transfected with control or APC siRNA. (D) Representative immunoblot for CK1α abundance in wt intestinal organoids maintained in regular niche factors alone (−) or supplemented with WNT3A and nicotinamide (Nico; 1 mM) to hyperactivate WNT signaling. (E) Morphology in intestinal organoids described in (D) treated with SSTC3 for 4 days. (F) TOPflash WNT reporter activity (left) in 293T cells after CK1α knockdown with SMARTpool siRNA (right) and treatment with vehicle or SSTC3 (100 nM for 24 hours). (G) Kaplan-Meier survival curves of patients with tumors that had relatively high or low CK1α expression (GSE17538; n = 125 patients per group; *P ≤ 0.05). (H) Schematic demonstrating the selectivity of CK1α activators for WNT-dependent tumors.