Abstract
Muscarinic acetylcholine receptors exert slow and prolonged synaptic effects in both vertebrate and invertebrate nervous systems. Through activation of G proteins, they typically decrease intracellular cAMP levels by inhibition of adenylate cyclase or stimulate phospholipase C and the turnover of inositol phosphates. In insects, muscarinic receptors have been credited with two main functions: inhibition of transmitter release from sensory neuron terminals and regulation of the excitability of motoneurons and interneurons. Our pharmacological studies with intact and behaving grasshoppers revealed a functional role for muscarinic acetylcholine receptors as being the basis for specific arousal in defined areas of the brain, underlying the selection and control of acoustic communication behavior. Periodic injections of acetylcholine into distinct areas of the brain elicited songs of progressively increasing duration. Coinjections of the muscarinic receptor antagonist scopolamine and periodic stimulations with muscarine identified muscarinic receptor activation as being the basis for the underlying accumulation of excitation. In contrast to reports from other studies on functional circuits, muscarinic excitation was apparently mediated by activation of the adenylate cyclase pathway. Stimulation of adenylate cyclase with forskolin and of protein kinase A with 8-Br-cAMP mimicked the stimulatory effects of muscarine whereas inhibition of adenylate cyclase with SQ22536 and of protein kinase A with H-89 and Rp-cAMPs suppressed muscarine-stimulated singing behavior. Activation of adenylate cyclase by muscarinic receptors has previously been reported from studies on membrane preparations and heterologous expression systems, but a physiological significance of this pathway remained to be demonstrated in an in vivo preparation.
Acetylcholine (ACh) is an important transmitter in the nervous systems of both vertebrates and invertebrates. It activates two structurally distinct classes of receptors named nicotinic and muscarinic ACh receptors (mAChRs) according to their activation by the distinguishing agonists nicotine and muscarine. Nicotinic AChRs are ligand-gated cation channels that, on activation, mediate rapid and short-lived depolarization. In contrast, mAChRs are coupled through G proteins to intracellular second messenger pathways, leading to signals that are slower in onset and longer in duration (for review, see refs. 1 and 2). Five different subtypes of mAChRs have been cloned from vertebrates that differ in their selectivity for agonists and antagonists, in their expression patterns within the central nervous system, in their coupling to specific G proteins, and thus in their effects on second messengers (1–3). Although mAChRs have been found in various insect nervous systems (for review, see refs. 4 and 5), only one subtype, from Drosophila, has been cloned to date (6, 7). Because the pharmacological classification of invertebrate mAChRs does not correlate well with that of vertebrates (8), relatively little is known about the diversity of receptor subtypes and their functional coupling to second messengers in individual invertebrate neurons (4, 5). The most extensive studies have been performed on sensory-to-interneuron synapses in cockroaches (9) and sensory-to-motoneuron synapses in larvae of tobacco hawkmoths (10). In both preparations, two functions, apparently served by different types of muscarinic receptors, have been identified. Presynaptic receptors, present on sensory terminals, inhibit transmitter release through inhibition of the adenylate cyclase pathway, depending on previous synaptic activity (9–11). Activation of postsynaptic receptors increases the excitability of motoneurons and interneurons, thereby causing a sensitization to presynaptic input (9, 10). In addition to these identified effects, global application of muscarinic agonists activates central pattern generating circuits for various insect behaviors such as locomotion (12, 13), chewing, and pharyngeal movements (5, 14). However, the neurons targeted by muscarinic agonists and the immediate effects they evoke are generally difficult to explore in behaving preparations.
A promising preparation in which to study subcellular mechanisms that contribute to the control of behavior is the singing behavior (stridulation) of gomphocerine grasshoppers. Grasshopper songs are genetically determined and not altered by learning processes. Many species generate distinguishable songtypes that serve as specific signals in the context of attracting partners for reproduction, courting, and agonistic behavior (for review, see ref. 15). Courtship songs in particular may include various pattern elements, the composition of which gradually changes with the progress of courting and increasing levels of excitation (16, 17). The central pattern generating circuits driving the sound-producing hindleg movements during stridulation are located in the third thoracic ganglion complex (18, 19) (Fig. 1). These thoracic pattern generators are activated through tonic discharges of descending protocerebral command neurons (20). In a given species, each of several identifiable types of command neurons controls one specific pattern of stridulatory hindleg movements whose performance is associated with a particular behavioral situation (21). Grasshopper stridulation can be stimulated by focal injections of cholinergic agonists into specific areas of the protocerebrum, the central body complex, and a small neuropil situated posterior and dorsal to it (16, 22). When stimulated by nicotinic agonists, stridulation is rapidly initiated and short-lived, whereas stimulation by muscarinic agonists produces slowly initiated, long-lived bouts of stridulation. This indicates that ACh is a natural transmitter, mediating excitation in the protocerebral circuits that control stridulatory behavior.
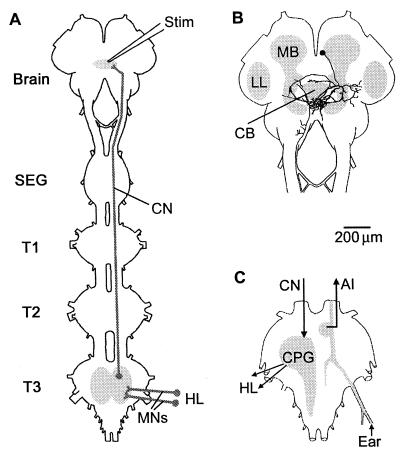
Figure 1.
Grasshopper central nervous structures involved in the control of stridulation and auditory information flow. (A) Schematic drawing of the head and the thoracic portion of the ventral nerve cord illustrating the site of pharmacological stimulation (Stim) in the supraesophageal ganglion (Brain), the cephalo-thoracic projection of the command neurons (CN), and the motor neurons (MNs) that excite the muscles of the hindlegs (HL) to produce the stridulatory movements. (B and C) The command neurons in the supraesophageal ganglion (B) have dense arborizations dorsal and posterior to the central body (CB). They (CN) activate the central pattern generating circuits (CPG) in the third thoracic ganglion (B) that generate the neuromuscular patterns underlying the stridulatory hindleg (HL) movements. Auditory receptor cells couple in T3 to ascending interneurons (AI) that relay the information to the lower lateral protocerebrum (LL), where the neural filters for pattern recognition are located. MB, mushroom body, SEG, subesophageal ganglion, and T1–T3 first to third thoracic ganglion.
In this publication, we present a more thorough investigation of the contribution of muscarinic excitation to the control of grasshopper singing behavior. Various membrane permeable substances known to interfere with the adenylate cyclase second messenger pathway were tested for their ability to mimic the stimulatory effects of muscarine or to suppress muscarine-stimulated singing behavior. The results suggest that mAChRs in the brain of grasshoppers mediate excitation by activation of adenylate cyclase, supporting previous studies on cell membrane preparations and heterologous expression systems in which a physiological role for this pathway was suggested (23–26). We further demonstrate by periodic stimulation with ACh and muscarine, by the use of specific antagonists, and by pharmacological interference with natural stimuli relevant to singing behavior, that the activity of the muscarinic system determines (i) the behavioral threshold for engagement in stridulation and (ii) the selection of song patterns associated with increasing arousal during the progress of courtship.
Materials and Methods
Animals.
Experiments were performed with adult male grasshoppers of the two species Omocestus viridulus (L.) and Chorthippus biguttulus (L.). Both species were caught in the vicinity of Göttingen (Germany) and kept in the laboratory for up to 2 wk. All studies were performed at room temperature (24–28°C).
Drugs.
The neuroactive drugs (ACh, muscarine, scopolamine, 8-Br-cAMP, 3-isobutyl-1-methylxanthine, and forskolin were obtained from Sigma–Aldrich; SQ22536 and Rp-cAMPs were obtained from Calbiochem, and H-89 was obtained from Biomol, Plymouth Meeting, PA) were dissolved in grasshopper saline (27) to give concentrations of 10−3 M. Forskolin and H-89 were first dissolved in DMSO to give a final concentration of 5% DMSO after saline was added. Control injections of DMSO solution to sites within the brain where muscarine reliably stimulated stridulation had no effect.
Experimental Design.
Grasshoppers were restrained with wax at their pronota, the head capsules opened dorsally, and the brains tipped forward to expose their dorsal surfaces. Drugs were applied through a capillary with two chambers, pulled to a single tip of ≈10–15 μm in diameter. The capillary was coupled to a pressure pump (model PV 820, WPI Instruments, Waltham, MA), enabling individual injections of small (1–3 nl) volumes of two different drugs to the same site within the brain. Stridulatory hindleg movements were recorded with optoelectronic cameras (28). Recordings of hindleg movements, sound, and the injection pulse were digitized and stored on a personal computer. The data were processed with the following tools: visual examination with the analysis program neurolab (29), generation of histograms with excel (Microsoft), statistical evaluation with prism (Graphpad, San Diego), and assembling of figures with photoshop (Adobe Systems, Mountain View, CA).
Results and Discussion
Previous pharmacological studies on gomphocerine grasshoppers established that species- and context-specific stridulation can be stimulated by injection of both nicotinic and muscarinic agonists into a particular region of the brain (16, 22). However, the functional role of mAChR-mediated excitation in the brain circuits that control specific singing behavior remained unclear because muscarinic effects are believed to depend on sustained release of ACh as it has previously been suggested for the activity of certain sensory afferents (5, 9, 11).
Stimulation of mAChRs Leads to Prolonged and Accumulating Excitation in the Control Circuits for Stridulation.
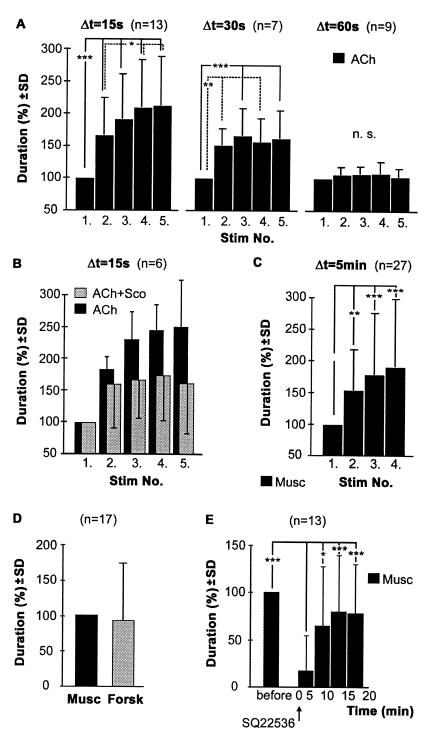
In one series of experiments, the injection capillary was placed at a site within the protocerebrum where repeated injections of ACh, the presumed natural excitatory transmitter, reliably elicited stridulatory behavior (Fig. 2A). With regular stimulus intervals of 15 s (Fig. 2A Left), the first of a series of identical ACh stimuli elicited periods of stridulation that were relatively short, compared to later injections. The following ACh stimuli led to gradually increasing durations of stridulation, which saturated after the third or fourth injection. Increased durations of stridulation were always associated with shorter latencies to the onset of stridulation (not shown). With stimulus intervals of 30 s, the overall increase of duration was less pronounced (Fig. 2A Center). ACh stimulation at intervals of 60 s failed to cause significant changes in the duration of stridulation (Fig. 2A Right). These results indicated that the ACh-induced excitation outlasts not only the stridulatory activity of 1–5 s but an interval of at least 30 s. With sufficiently short interstimulus intervals, this leads to an accumulation of excitation, causing faster onset of prolonged stridulation in response to the following ACh stimulus.
Figure 2.
Activation of mAChR mediates long lasting and cumulative excitation. (A) Repeated injections of ACh into the central protocerebrum released stridulation of progressively increasing duration. Changes were most pronounced with stimulus intervals of 15 s (Left), still highly significant with intervals of 30 s (Center), but absent with stimulus intervals of 60 s (Right) (one-way ANOVA for repeated measures). (B) Reducing the activation of mACh receptors by coinjection of the muscarinic receptor antagonist scopolamine caused a highly significant (two-way ANOVA, P = 0.0001) reduction in the progressive increase of stridulation in comparison to ACh alone. Data were obtained from six animals, in which both treatments were repeatedly tested at identical injection sites. (C) Increases in the duration of stridulation were also seen with repeated injections of muscarine at intervals of 5 min. (D) Injections of muscarine and forskolin stimulated stridulation of equal duration. (E) Injection of the adenylate cyclase inhibitor SQ22536 reversibly reduced the duration of stridulation stimulated by identical muscarine injections (P < 0.001, one-way ANOVA for repeated measures). All drugs were used at concentrations of 10−3 M in the pipet. The mean duration of stridulation at a specific stimulation site was set to 100% as follows: using the first stimulation of a series (A–C), muscarine alone (D), and muscarine before SQ22536 (E).
To determine the nature of this cumulative excitation, activation of mAChRs was prevented by coinjection of the general muscarinic receptor antagonist scopolamine (4), which has been shown to antagonize muscarine-stimulated stridulatory behavior (22). Double-barrel injection capillaries were inserted into areas within the protocerebrum where repeated injections of ACh (Δt = 15 s) led to the characteristic changes in the duration of stridulation (Fig. 2B, black columns). A mixture of ACh and scopolamine injected to identical sites reliably elicited stridulation of similar durations as those seen in the first injections of a series with ACh alone. However, the increase of durations (Fig. 2B, gray columns) was significantly reduced (P = 0.0001; two-way ANOVA), suggesting a partial suppression of muscarinic excitation underlying this phenomenon by the coinjected competitive antagonist. When instead of ACh the mAChR agonist muscarine was used for stimulation, the onset of stridulation was slower and its duration prolonged (22). As with ACh, repeated injections of muscarine (Fig. 2C, Δt = 5 min) led to progressively prolonged stridulation and faster onset of activity (not shown). The intervals between individual muscarine stimuli could be as long as 10 min and still produce cumulative excitation, indicating a prolonged presence of muscarine at the receptors and/or a stronger and more persistent activation of the second messenger pathway coupling to this subtype of mAChR.
Muscarinic Effects Are Mediated by Activation of the Adenylate Cyclase Second Messenger Pathway.
To explore the basis of the long-lasting effects, membrane permeable substances known to interfere with intracellular second messenger pathways were injected at sites where muscarine reliably stimulated stridulatory behavior. Direct activation of adenylate cyclase by forskolin (30) stimulated stridulation with similar temporal characteristics as muscarine injected at identical sites within the protocerebrum (Fig. 2D). Similarly, injections of 8-Br-cAMP, an analog of endogenously generated cAMP (31), and 3-isobutyl-1-methylxanthine, an inhibitor of phosphodiesterase (32), were also effective in stimulating stridulation (not shown). These results suggested that the second messenger cAMP contributes to the generation of excitation in the cephalic circuits that initiate stridulation. A linkage between stimulation of mAChRs and activation of the adenylate cyclase pathway was demonstrated with the following experiment. A capillary was placed in the central protocerebrum where periodic (Δt = 5 min) injections of muscarine elicited stridulatory activity of similar duration. Interposed injections to the same site within the brain of SQ22536, an inhibitor of adenylate cyclase (33), reversibly reduced the overall duration of stridulation (P < 0.001, 0–5 min after application, ANOVA for repeated measures) that was induced by identical muscarine stimuli (Fig. 2E). Similar results were obtained with H-89 and Rp-cAMPs (not shown), two substances that reduce the activation of protein kinase A by cAMP (31, 34). Because muscarine-stimulated stridulation was completely suppressed in some of these experiments (e.g., in 9 of 13 experiments with SQ22536), activation of adenylate cyclase leading to the formation of the second messenger cAMP appeared to be necessary for the mediation of the excitatory muscarinic effects.
Activation of mAChRs Decreases the Behavioral Threshold for Engagement in Singing Behavior in Response to Natural Stimuli.
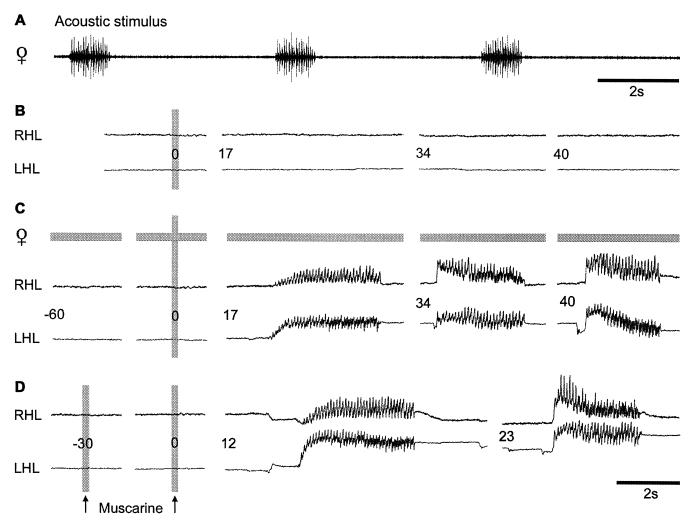
A functional contribution of muscarinic excitation to the cephalic control of stridulation was not obvious because muscarinic effects, in contrast to transient activation of nicotinic receptors, are thought to depend on sustained release of ACh from presynaptic sources like sensory terminals (5, 10). Although direct activation by auditory afferents of the cephalic circuits that control stridulation can be excluded (35, 36) (Fig. 1), songs of conspecific females (Fig. 3A) have been demonstrated to effectively stimulate males to answer, engage in song dialogues, and approach the female (37). During our pharmacological experiments, males of the species Ch. biguttulus were fixed to a holder and never responded spontaneously to female songs presented via a loudspeaker. A similar decreased responsiveness has been observed in other studies with restrained animals (38). A small amount of muscarine, not sufficient to induce stridulation (Fig. 3B), was injected into the protocerebrum at a site where injections of larger volumes of muscarine elicited the behavior (not shown). This small amount of muscarine was administered every 10 min throughout the entire experiment to assure the same basal muscarinic excitation in the protocerebral circuitry. Beginning at 1 min before every second trial, female songs were presented. It was only in connection with these acoustic stimuli that the standard muscarine injection elicited 2–3 song sequences in the male (Fig. 3C). The stimulating effect of the female song that contributed to excite the male beyond its response threshold could be replaced by a preceding injection of muscarine that was given 30 s in advance to the test pulse (Fig. 3D). These results indicate that excitation mediated by both stimuli, the female song and the injected muscarine, converges at some point within the cephalic control circuit and accumulates to release stridulatory behavior. Both stimuli elevate the basal level of excitation or arousal, thereby priming the male to stridulate in response to an additional stimulatory input. Simply speaking, the mAChR-mediated excitation provides a substrate for motivation in the protocerebral control center for stridulation. Although some evidence exists that the integrated sensory input from the female song may lead to activation of the muscarinic system, activation of a different pathway that contributes to the specific arousal cannot be excluded at this point.
Figure 3.
Stimulation of male Ch. biguttulus with muscarine and song of a conspecific female. (A) Recording of a female song sequence. During acoustic stimulation, this song sequence was presented every 5 s at an intensity of 60 dB SPL (relative to 2 · 10−5 Pa). (B) Injection of muscarine to a specific site within the protocerebrum did not release stridulation. Similarly, songs of a conspecific female had no effect on the restrained male (not shown). (C) In contrast, a combination of female songs, starting 60 s before pharmacological stimulation and the unchanged muscarine stimulus elicited stridulation resembling the natural song dialogues observed in this species. (D) The excitation provided by the auditory stimulus could be mimicked by a second injection of muscarine, 30 s before the test pulse. RHL and LHL, right and left hind leg.
The Amount of mAChR Mediated “Basal” Excitation Determines the Selection of Stridulation Patterns Associated with a Certain Behavioral Situation.
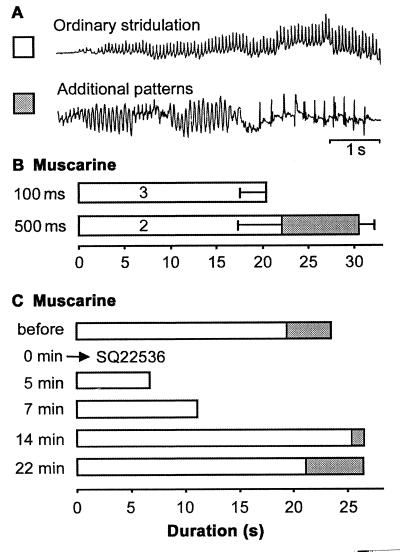
In addition to lowering the behavioral threshold, muscarinic excitation with its characteristics described in Fig. 2, also may determine the use of song patterns that emerge during later stages of courtship and are associated with increasing arousal (16, 17). Courtship songs of O. viridulus consist of several sequences but only the last two or three sequences immediately preceding a mating attempt are supplemented by short parts of two additional movement and sound patterns (Fig. 4A), indicating the highest level of arousal. Two types of pharmacological experiments suggested that the composition of courtship songs may depend on the level of mAChR- and adenylate cyclase- mediated excitation. (i) Increasing the amount of muscarine administered to a fixed site in the protocerebrum resulted in two effects: a prolonged duration of induced stridulatory activity and the appearance of the additional patterns related to the final stages of courtship (Fig. 4B). (ii) When complete courtship sequences that included the additional patterns were initiated by identical muscarine stimuli at regular intervals of Δt = 5 min, interposed injections of SQ22536 reduced the performance of the ordinary pattern but completely abolished the additional patterns (Fig. 4C). With the effect of the adenylate cyclase inhibitor diminishing, the additional patterns associated with high courtship intensity reappeared only after the duration of the part consisting of the ordinary pattern had been restored to its level before SQ22536 interference. Although the amount of stridulatory activity appeared to depend on the duration of suprathreshold excitation in the control circuit, the selection and performance of patterns related to higher arousal toward the end of courtship stridulation seemed to be determined by a second, more elevated threshold. This second threshold may only be passed with the contribution of substantially elevated levels of mAChR-mediated excitation that accumulates during the first part of courtship in the presence of the conspecific female.
Figure 4.
Muscarinic excitation contributes to the selection of song patterns. (A) Hindleg movements of courtship stridulation in O. viridulus. The additional patterns are only included during the final stages of courtship. (B) Increased amounts of muscarine not only prolonged the duration of stridulation but also stimulated the additional patterns characteristic for the final phase of courtship (100 ms/500 ms duration of pressure pulses). (C) Inhibition of adenylate cyclase by SQ22536 reduced the overall duration of courtship stridulation and completely eliminated the additional patterns associated with higher courtship intensity. These patterns only reappeared after the overall duration of stridulation had already been reestablished.
In summary, the results presented above together with a detailed knowledge of grasshopper stridulatory behavior lead to the following picture: ACh, released from yet unidentified presynaptic brain neurons, initiates stridulatory behavior through its actions on both nicotinic and muscarinic AChRs. Stimulation of mAChRs leads to prolonged excitation based on the activation of adenylate cyclase and increased levels of the second messenger cAMP. Considerable excitation, mediated by mAChRs, accumulates only with repeated or persistent release of ACh from presynaptic sources, because of suitable sensory input such as the song or the presence of a potential reproductive partner. During prolonged courtship stridulation in the immediate vicinity of the female, mAChR-mediated arousal progressively builds up to a certain level, at which additional song patterns may be included and a mating attempt initiated. Our studies in the brain of grasshoppers suggest a functional role for mAChRs in the selection and control of specific behaviors relevant to a certain situation. This function supplements previously described effects of mAChRs, that modulate presynaptic transmitter release and postsynaptic excitability at sensory-to-motoneuron or sensory-to-interneuron synapses (9–11). Pharmacological interference with the adenylate cyclase/cAMP second messenger pathway indicated that mAChRs in the central protocerebrum of grasshoppers activate adenylate cyclase. This demonstrates a physiological significance for this pathway, that previously has only been described for heterologously expressed mAChRs, tissue extracts and membrane preparations (23–26).
Acknowledgments
We thank Dr. Geoffrey Ganter for his thoughtful comments on this paper. The study was supported by Grant EL 35/19-1 of the Deutsche Forschungsgesellschaft (to N.E. and R.H.) and the graduate program “Organization and Dynamics of Neuronal Networks” (to B.W.).
Abbreviations
- ACh
acetylcholine
- mAChR
muscarinic acetylcholine receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 9468.
References
- 1.Felder C C. FASEB J. 1995;9:619–625. [PubMed] [Google Scholar]
- 2.Jones S V. Life Sci. 1993;52:457–464. doi: 10.1016/0024-3205(93)90302-j. [DOI] [PubMed] [Google Scholar]
- 3.Bonner T I. Trends Neurosci. 1989;12:148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- 4.Hannan F, Hall L M. In: Comparative Molecular Neurobiology. Pichon Y, editor. Basel: Birkhäuser; 1993. pp. 98–145. [Google Scholar]
- 5.Trimmer B A. Trends Neurosci. 1995;18:104–111. [PubMed] [Google Scholar]
- 6.Onai T, Fitzgerald M G, Arakawa S, Gocayne J D, Urquhart D A, Hall L M, Fraser C M, McCombie W R, Venter J C. FEBS Lett. 1989;255:219–225. doi: 10.1016/0014-5793(89)81095-6. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro R A, Wakimoto B T, Subers E B, Nathanson N M. Proc Natl Acad Sci USA. 1989;86:9039–9043. doi: 10.1073/pnas.86.22.9039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qazi S, Proulx D, Trimmer B A. Insect Biochem Mol Biol. 1996;26:721–732. doi: 10.1016/s0965-1748(96)00042-2. [DOI] [PubMed] [Google Scholar]
- 9.LeCorronc H, Hue B. J Exp Biol. 1993;181:257–278. [Google Scholar]
- 10.Trimmer B A, Weeks J C. J Neurophysiol. 1993;69:1821–1836. doi: 10.1152/jn.1993.69.6.1821. [DOI] [PubMed] [Google Scholar]
- 11.Knipper M, Breer H. J Comp Biochem Physiol C. 1988;90:275–280. [Google Scholar]
- 12.Ryckebush S, Laurent G. J Neurophysiol. 1993;69:1583–1595. doi: 10.1152/jn.1993.69.5.1583. [DOI] [PubMed] [Google Scholar]
- 13.Büschges A, Schmitz J, Bässler U. J Exp Biol. 1995;198:435–456. doi: 10.1242/jeb.198.2.435. [DOI] [PubMed] [Google Scholar]
- 14.Gorczyca M G, Budnik V, White K, Wu C F. J Neurobiol. 1991;22:391–404. doi: 10.1002/neu.480220407. [DOI] [PubMed] [Google Scholar]
- 15.Elsner N. In: Neural Basis of Behavioural Adaptations. Schildberger K, Elsner N, editors. Stuttgart: Gustav Fischer; 1994. pp. 167–194. [Google Scholar]
- 16.Heinrich R, Wenzel B, Elsner N. J Comp Physiol A. 2001;187:155–169. doi: 10.1007/s003590100188. [DOI] [PubMed] [Google Scholar]
- 17.Helversen O v. Zool Jb Syst. 1986;113:319–342. [Google Scholar]
- 18.Ronacher B. J Comp Physiol A. 1989;164:723–736. [Google Scholar]
- 19.Heinrich R, Elsner N. J Comp Physiol A. 1997;180:257–269. [Google Scholar]
- 20.Hedwig B. J Neurophysiol. 1994;72:2015–2025. doi: 10.1152/jn.1994.72.4.2015. [DOI] [PubMed] [Google Scholar]
- 21.Hedwig B, Heinrich R. J Comp Physiol A. 1997;180:285–294. [Google Scholar]
- 22.Heinrich R, Hedwig B, Elsner N. J Exp Biol. 1997;200:1327–1337. doi: 10.1242/jeb.200.9.1327. [DOI] [PubMed] [Google Scholar]
- 23.Enyedi P, Fredholm B B, Lundberg J M, Änggard A. Eur J Pharmacol. 1982;79:139–143. doi: 10.1016/0014-2999(82)90586-6. [DOI] [PubMed] [Google Scholar]
- 24.Olianas M C, Onali P. J Neurochem. 1992;58:1723–1729. doi: 10.1111/j.1471-4159.1992.tb10046.x. [DOI] [PubMed] [Google Scholar]
- 25.Felder C C, Kanterman R Y, Ma A L, Axelrod J. J Biol Chem. 1989;264:20356–20362. [PubMed] [Google Scholar]
- 26.Jones S V, Heilman C J, Brann M R. J Mol Pharmacol. 1991;40:242–247. [PubMed] [Google Scholar]
- 27.Clements A N, May T E. J Exp Biol. 1974;60:673–705. doi: 10.1242/jeb.60.3.673. [DOI] [PubMed] [Google Scholar]
- 28.Helversen O v, Elsner N. J Comp Physiol A. 1977;122:53–64. [Google Scholar]
- 29.Hedwig B, Knepper M. J Neurosci Methods. 1992;45:135–148. doi: 10.1016/0165-0270(92)90051-e. [DOI] [PubMed] [Google Scholar]
- 30.Seamon K B, Wetzel B. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:91–99. [PubMed] [Google Scholar]
- 31.Lundquist C T, Nässel D R. J Neurobiol. 1997;33:297–315. doi: 10.1002/(sici)1097-4695(199709)33:3<297::aid-neu8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.Trimmer B A, Qazi S. J Neurochem. 1996;66:1903–1913. doi: 10.1046/j.1471-4159.1996.66051903.x. [DOI] [PubMed] [Google Scholar]
- 33.Goldsmith B A, Abrams T W. Proc Natl Acad Sci USA. 1991;88:9021–9025. doi: 10.1073/pnas.88.20.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith W A, Varghese A H, Healy M S, Lou K J. Insect Biochem Mol Biol. 1996;26:161–170. doi: 10.1016/0965-1748(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 35.Boyan G S. J Comp Physiol A. 1983;151:499–513. [Google Scholar]
- 36.Bauer M, Helversen O v. J Comp Physiol A. 1987;161:95–101. [Google Scholar]
- 37.Helversen D v, Helversen O v. J Comp Physiol A. 1997;180:373–386. [Google Scholar]
- 38.Krasne F B, Wine J J. J Exp Biol. 1975;63:433–450. doi: 10.1242/jeb.63.2.433. [DOI] [PubMed] [Google Scholar]