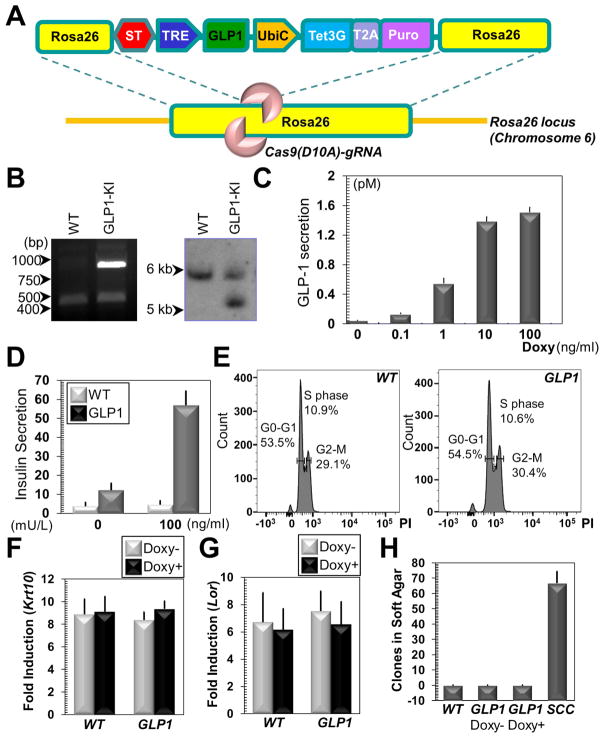
Figure 1. Engineering GLP1-producing skin epidermal progenitor cells with CRISPR.
(A) The targeting vector contains two Rosa26 homology arms, flanking the expression cassette for GLP1 (driven by TRE/tet-on promoter). Tet3G (tetracycline transactivator) protein and a selection marker (Puro) are separated by a self-cleavable peptide T2A and driven by a constitutive promoter UbiC (Ubiquitin C promoter). ST: transcriptional stop signal (ST). (B) Integration of the targeting vector into Rosa26 locus is verified by PCR (left panel) and southern blotting (right panel). (C) Secretion of GLP1 in cell culture medium is determined by ELISA (enzyme-linked immunosorbent assay) upon stimulation with doxycycline (Doxy). All error bars represent standard deviation unless otherwise specified. Sample size n=3 independent experiments. (D) Conditioned medium is collected and used to treat starved insulinoma cells. Secretion of insulin in vitro is determined by ELISA. n=4 independent experiments. (E) FACS (fluorescence activated cell sorting) demonstrates similar cell cycle profiles for WT (wild type) and GLP1-expressing epidermal progenitor cells after doxycycline treatment. PI: propidium iodine. (F–G) Western blotting analysis of early (F) and late (G) differentiation marker expression in WT and GLP1-expressing cells upon calcium shift. Band intensity was determined by densitometry and fold of induction is quantified. Krt10: keratin 10; Lor: loricrin. n=4 independent experiments. (H) WT cells or GLP1 cells with or without doxycycline treatment are tested for anchorage independent growth in soft agar. SCC: mouse squamous cell carcinoma cancer initiating cells. n=3 independent experiments.