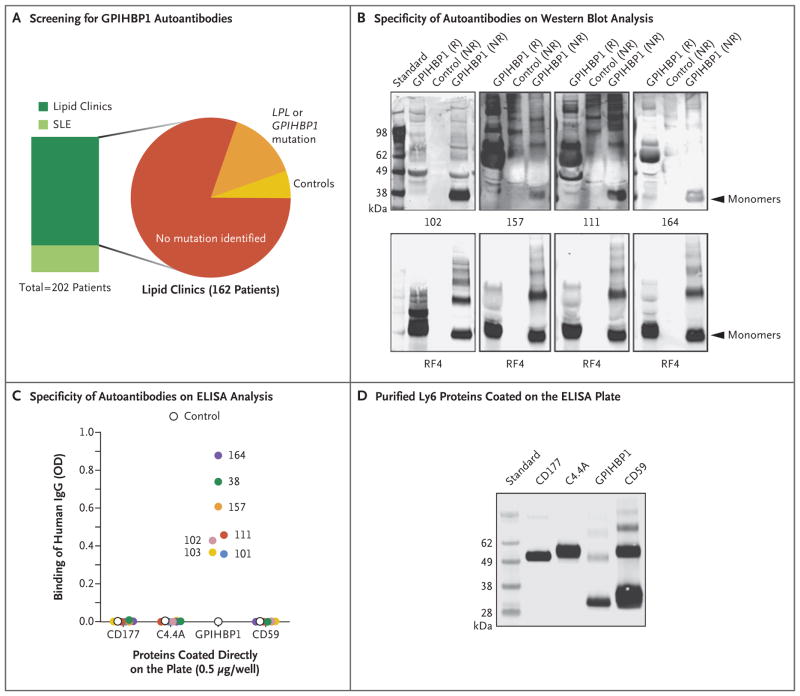
Figure 3. Identification of Four Additional Plasma Samples with GPIHBP1 Autoantibodies.
Panel A summarizes the plasma samples screened for GPIHBP1 autoantibodies in 202 deidentified archived plasma samples from lipid clinics or a rheumatology clinic in which patients with systemic lupus erythematosus (SLE) were being treated. Forty patients with SLE, half of whom were receiving immunosuppressive therapy, were included; 10 of those patients had plasma triglyceride levels ranging from 350 to 750 mg per deciliter (4.0 to 8.5 mmol per liter). The 162 patients from various lipid clinics included 130 patients who had hypertriglyceridemia with no known genetic cause, 23 patients who had hypertriglyceridemia and a mutation in GPIHBP1 or LPL, and 9 controls. (Details are provided in Table S1 in the Supplementary Appendix.) Panel B shows Western blot analysis demonstrating that the immunoglobulins in plasma samples obtained from Patients 102, 157, 111, and 164 (containing 20 AU of GPIHBP1 autoantibody per milliliter) bind preferentially to nonreduced human GPIHBP1. The lower panels show the same blots incubated with the human GPIHBP1–specific monoclonal antibody RF4 (4 μg per milliliter). The monoclonal antibody RF4, which binds to reduced and nonreduced forms of GPIHBP1 and to GPIHBP1 multimers, was used as a loading control. The control culture medium was obtained from S2 cells that do not express human GPIHBP1. Panel C shows the results from ELISA analyses revealing that the immunoglobulin (IgG) in plasma samples obtained from Patients 38, 101, 102, 103, 111, 157, and 164 bind to wells of an ELISA plate coated with purified human GPIHBP1 but not to wells coated with other human Ly6 proteins (CD177, C4.4A, and CD59) (see Methods Section S4 in the Supplementary Appendix). The dilution was 1:12,500 for the sample obtained from Patient 102 and 1:500 for the other samples. A control plasma sample did not bind to any of the Ly6 proteins. The optical density (OD) was measured at a wavelength of 450 nm. Panel D shows Western blot analysis (performed with the use of monoclonal antibody R2416) of purified Ly6 proteins (CD177, C4.4A, GPIHBP1, and CD59) that had been fractionated according to size by means of SDS-PAGE. All four proteins had an amino-terminal urokinase-type plasminogen activator receptor (uPAR) tag that could be detected with monoclonal antibody R24. This Western blot analysis documents that similar amounts of all four Ly6 proteins were used to coat 96-well plates for the ELISA analysis shown in Panel C.