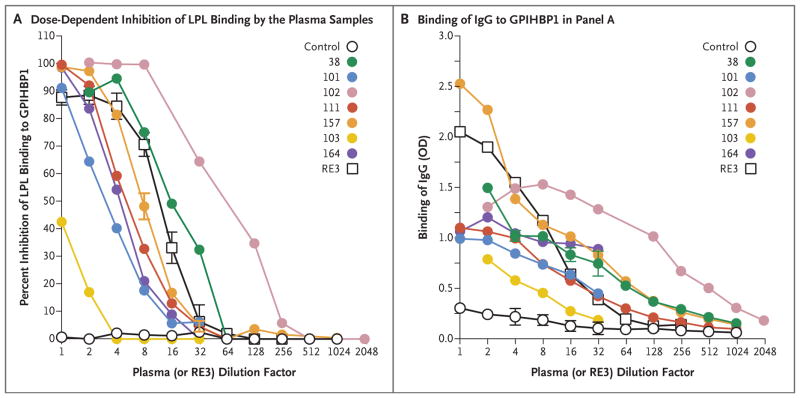
Figure 5. Blocking of Binding of Lipoprotein Lipase to GPIHBP1 by Autoantibodies in a Dose-Dependent Manner.
Panel A shows the results of a solid-phase assay of binding of lipoprotein lipase to GPIHBP1 (see Methods Section S5 in the Supplementary Appendix). Briefly, ELISA plates were coated with the monoclonal antibody R24 and incubated with uPAR-tagged human GPIHBP1, followed by an overnight incubation with serial dilutions of human plasma samples or the GPIHBP1-specific monoclonal antibody RE3. The next day, after incubating the plates with V5-tagged human lipoprotein lipase, the amount of GPIHBP1-bound lipoprotein lipase was detected with a horseradish peroxidase (HRP)–labeled V5 antibody and compared with the amount of bound lipoprotein lipase in the absence of human plasma or monoclonal antibody RE3 (set at 100% binding). These ELISA studies showed that GPIHBP1 autoantibodies in the plasma of six adult patients with chylomicronemia (Patients 38, 101, 102, 111, 157, and 164) and the plasma of an infant of Patient 102 (Patient 103) blocked binding of lipoprotein lipase to GPIHBP1, as did the monoclonal antibody RE3. (The 1:1 dilution for monoclonal antibody RE3 corresponds to 20 μg per milliliter.) A control plasma sample did not block binding of lipoprotein lipase to GPIHBP1. Panel B shows the amount of IgG binding to GPIHBP1 for each of the dilutions tested in Panel A. Plasma samples obtained from Patients 38, 102, and 157 had the highest titers of GPIHBP1 autoantibody.