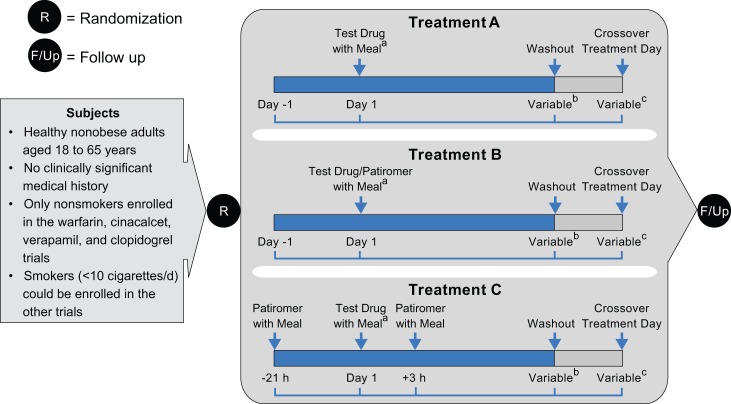
Figure 1.
Design of in vivo drug interaction studies: open-label, randomized, 3-way crossover. Treatment A—Each victim drug was administered alone within 30 minutes after the start of a standard breakfast (day 1), except for levothyroxine administered within 40 minutes before breakfast. Treatment B—Victim drugs were administered together with patiromer. Each victim drug was given within 30 minutes after the start of a standard breakfast and patiromer within 10 minutes after the victim drug (day 1), except levothyroxine, administered at 40 minutes before breakfast followed by patiromer administered with breakfast. Treatment C—Victim drugs were administered between 2 patiromer doses. The first dose of patiromer was administered within 30 minutes after the start of a standard lunch (day −1). Each of the victim drugs was administered 21 hours after the first patiromer dose and within 30 minutes of a standard breakfast on day 1 (except levothyroxine, which was administered at 40 minutes prior to standard breakfast). The second patiromer dose was administered 3 hours after the victim drug and within 30 minutes after the start of a standard lunch. aPatiromer and the victim drugs were always administered with meals, except for levothyroxine, which was given on empty stomach, within 40 minutes prior to the meal. bDuration from administration of victim drug to final draw of blood for pharmacokinetics (PK) analysis of drug concentration varied, generally depending on the PK characteristics of the victim drug. Time from the administration of victim drug (day 0) to the beginning of washout (hours): warfarin (168); verapamil (36), lithium (96), trimethoprim (60), amlodipine (144), cinacalcet (144), furosemide (12), metoprolol (36), clopidogrel (32), ciprofloxacin (24), metformin (24), and levothyroxine (48). cDuration of washout before the administration of victim drug after crossover from previous treatment varied, depending on the PK characteristics of the drug. Between-treatment washout periods (in days): warfarin (≥19), verapamil (≥5), lithium (≥10), trimethoprim (≥4), amlodipine (≥14), cinacalcet (≥10), furosemide (≥4), metoprolol (≥5), clopidogrel (≥3), ciprofloxacin (≥3), metformin (≥4), and levothyroxine (≥35).