Abstract
Hyaluronic acid (HA) has been a treatment modality for patients with knee osteoarthritis (OA) for many years now. Since HA was first introduced for the treatment of painful knee OA, much has been elucidated regarding both the etiology of this disease and the mechanisms by which HA may mitigate joint pain and tissue destruction. The objectives of this article are to (1) describe the etiology and pathophysiology of OA including both what is known about the genetics and biochemistry, (2) describe the role of HA on disease progression, (3) detail the antinociceptive and anti-inflammatory actions of HA in OA, and (4) present evidence of disease-modifying effects of HA in the preservation and restoration of the extracellular matrix. These data support that HA is not only just a simple device used for viscosupplementation but also a biologically active molecule that can affect the physiology of articular cartilage.
Keywords: Knee, osteoarthritis, hyaluronic acid, mechanism of action
Introduction
Over the past several decades, osteoarthritis (OA) of the knee and hip has emerged as the 11th leading cause of global disability.1 Among these 2 conditions, the prevalence of knee OA exceeds that of hip OA by several fold and it is estimated that nearly 1 in every 2 people will develop symptomatic knee joint OA by the age of 85 years.1–3 In the United States alone, more than 9 million adults have symptomatic OA of the knee.4 In particular, it is the senior population that is disproportionately affected, with 37.4% of adults more than the age of 60 years displaying radiographic evidence of this condition. Approximately, a third of these individuals (12.1%) suffer significantly from symptoms of pain and disability.5
Despite the high prevalence of knee OA in those more than 60 years in the United States, less than 2% of this population has a knee joint replacement, and the mean duration of time from disease onset to arthroplasty is 19 years.5,6 Such statistics underscore the continued importance of exploring and optimizing nonoperative treatments, such as hyaluronan (HA), which may have the potential to influence the biology of OA and ultimately improve the quality of life for millions of people having OA of the knee.
Hyaluronan has been used in thousands of patients for the treatment of painful OA of the knee, and its efficacy for this indication is supported by results from multiple clinical trials.7–11 Despite being classified in the United States, Europe, and other countries as a medical device to treat knee OA via intra-articular injection, it has been recognized that HA preparations may provide clinical benefit beyond boundary lubrication and shock absorption, via biochemical and genetic modifications that can attenuate nociceptive responses and blunt inflammation associated with OA.12 Since HA was first introduced for the treatment of painful knee OA, much has been elucidated regarding both the etiology of this disease and the mechanisms by which HA may mitigate joint pain and tissue destruction.
The objectives of this article are to (1) describe the etiology and pathophysiology of OA, (2) describe the role of HA on disease progression, (3) detail the antinociceptive and anti-inflammatory actions of HA in OA, and (4) present evidence of disease-modifying effects of HA in the preservation and restoration of the extracellular matrix (ECM).
Etiology and Pathophysiology of OA
Genetics and epigenetics
The etiology of OA is complex and involves both hereditary factors and alteration of gene expression within chondrocytes due to environmental and mechanical factors. Approximately 50% of the risk of developing OA is heritable, but only a few loci have been significantly associated with OA in genome-wide association studies.13 This may be due to epigenetic modifications, such as DNA methylation, histone modifications, and noncoding RNA, that can remodel chromatin and alter gene expression, thereby contributing to the development of OA.13 Environmental factors and mechanical stress have been shown to induce epigenetic changes within different cell types leading to pathology.14 In the field of vascular biology, cells that are constantly exposed to fluid shear stress and cyclic stretch from blood pressure undergo heritable genetic modifications that can influence physiology and pathophysiology.14 Similarly, the joint environment is subject to constant pressure and sheer stress, which may lead to heritable changes in chondrocytes and synoviocytes via mechanotransduction pathways. The link between epigenetic changes and OA continues to be investigated and connections continue to be further elucidated. For example, the OA disease state has been associated with the methylation status of promoter regions responsible for expression of cartilage-degrading proteins such as matrix metalloproteinases (MMPs) and inflammatory molecules.15,16 Given the central role of proteinases and inflammatory molecules in the progression of OA, exploring the mechanisms underlying their increased expression and their cartilage-destroying pathophysiology is paramount to understanding the therapeutic effects of interventions such as HA.
Proteinases
Expression
Degradation of the ECM is a central feature of OA. In the OA joint environment, catabolic proteinases act on aggrecan and collagen, 2 essential components of the ECM.17 Polymorphisms in genes encoding MMP-1 and MMP-3 and A Disintegrin and Metalloprotease (ADAM)-12 have been shown to be associated with increased risk for the development of OA.18,19
Epigenetic changes in the regulation of genes encoding MMPs within synoviocytes and chondrocytes may also be involved in the development of OA. Significant promoter region demethylation of genes encoding MMP-3, MMP-9, and MMP-13, as well as A Disintegrin And Metalloproteinase with Thrombospondin Motifs (ADAMTS) 4, was found in chondrocytes extracted from patients with OA.20 These genes were also found to have methylated promoter regions in cartilage samples of patients without OA but who had suffered a femoral neck fracture.20 The demethylation positively correlated with the expression of these degrading enzymes.20 Reduced methylation of specific cytosine-phosphate-guanine (CpG) sites within the promoter region of MMP-13 and ADAMTS4 resulted in increased expression of these genes in the chondrocytes of patients with advanced OA and is consistent with the destructive effects of the enzymes that they encode.21 Epigenetic derepression associated with DNA methylation loss has also been demonstrated in chondrocytes affecting genes encoding MMP-3 and MMP-9.22
Pathophysiology of proteinases
Multiple studies have demonstrated increased levels of proteinases and aggrecanases in patients with OA.17 These enzymes cleave ECM proteins, including aggrecan and collagen, which may lead to chondral surface and structural damage.17 Hyaluronidase and reactive oxygen species (ROS) degrade HA into low-molecular-weight (LMW) fragments, and increased levels of LMW HA fragments are characteristic of later stage OA.23–25 Cross talk between subchondral bone osteoblasts and articular cartilage chondrocytes in OA alters the expression and regulation of a number of genes, including ADAMTS5, ADAMTS4, MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, and MMP-13. These effects are mediated by the mitogen-activated protein kinase/extracellular signal–regulated kinase 1/2 signaling pathways.26 Indirect coculture of OA subchondral bone osteoblasts with normal articular cartilage chondrocytes resulted in a significant increase in the expression of ADAMTS5, ADAMTS4, MMP-2, MMP-3, and MMP-9, whereas coculture of OA articular cartilage chondrocytes led to increased MMP-1 and MMP-2 expression in normal subchondral bone osteoblasts.26
The increased expression of MMPs in chondrocytes from patients with OA, coupled with a growing understanding of the pathways involved in upregulation of these enzymes, has made them targets for OA therapies.27 Inhibition of MMP-13 may be of particular benefit due to its specific expression in the cartilage of patients with OA but not normal adult cartilage samples. Development of MMP-13–specific inhibitors has the potential to avoid the musculoskeletal side effects that have been associated with broad-spectrum MMP blockers.28
Pro-inflammatory molecules
Expression
Inflammation plays a central role in the development and progression of OA, and there is a clear link between the progression of cartilage damage and the presence of a reactive or inflammatory synovium.29 Multiple inflammatory mediators are involved in OA, including interleukin (IL)-1β, IL-6, IL-15, IL-17, IL-18, IL-21, and tumor necrosis factor α (TNF-α).29 These cytokines act on multiple receptors, including CD44, HA-mediated motility receptor (RHAMM), and toll-like receptors (TLRs).30,31
The presence of inflammatory mediators is explained, at least in part, by increased genetic expression. Demethylation of specific CpG sites of the proximal IL-1β promoter in chondrocytes obtained from human cartilage has been correlated with increased expression levels for this gene.16 MicroRNAs also modify gene expression; it has been shown that miR-149 is downregulated in OA chondrocytes and that this decrease is associated with increased expression of the genes for pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6.32
Pathophysiology of inflammation
The cascade of inflammatory cytokines and other destructive molecules in OA has been well defined. Both IL-1β and TNF-α play key roles in cartilage destruction and could be considered as the initiators of the inflammatory cascade.29 IL-1β and TNF-α, produced by chondrocytes, mononuclear cells, osteoblasts, and synovial tissues, stimulate the production of many other inflammatory and catabolic molecules.29 IL-1β binds to receptors on chondrocytes and synovial cells and induces MMP synthesis. It also increases chondrocyte production of ADAMTS.33 In addition, IL-1β increases nitric oxide (NO) synthesis and decreases expression of the antioxidant enzymes that scavenge ROS, including superoxide dismutase, catalase, and glutathione peroxidase. The net effect of these changes may be an acceleration of the damaging effects of oxygen radicals on cartilage.29,34
Stimulation of chondrocytes by IL-1β also leads to the expression of TNF-α, which has many downstream pro-inflammatory effects.29,33 Tumor necrosis factor α suppresses the synthesis of proteoglycans and type II collagen in chondrocytes; stimulates the release of MMP-1, MMP-3, and MMP-13; and increases the production of IL-6, IL-8, monocyte chemotactic protein, and chemokine ligand 5.29
It has also been shown that IL-6, interferon-inducible protein 10, macrophage-derived chemokine, platelet-derived growth factor AA, and regulated on activation, normal T-cell expressed and secreted levels are higher in the synovial fluid from patients with OA as compared with that from normal controls (P < .001).35 Leptin, macrophage inflammatory protein 1β, and soluble CD40 levels are also elevated in synovial fluid from patients with OA vs that from normal subjects (P < .05).35
Interactions with these inflammatory mediators are the mechanism by which HA modifies the progression of OA. A detailed understanding of such interactions, as discussed in the following section, unequivocally highlights that the effects of HA extend beyond those of a “medical device” that simply lubricates and provides shock absorption.
Role of HA on Disease Progression
Hyaluronan is a naturally occurring ECM molecule found in synovial fluid and is fairly ubiquitous throughout the body.36 It is a high-molecular-weight (HMW) glycosaminoglycan that is present at high levels in cartilage and synovial fluid and is believed to play an important role in joint lubrication.37 Hyaluronan also complexes with lubricin, a glycoprotein, to form a network that creates a boundary lubricant that decreases friction force and greatly reduces wear damage on rubbing/shearing surfaces.37 The lubrication provided by HA and lubricin is adaptive in that HA diffuses out of the cartilage during joint compression and becomes mechanically trapped at the joint interface by a constricted collagen pore network, thereby forming HA-lubricin complexes.37 Hyaluronan also endows synovial fluid with its viscoelastic properties.38
Altered characteristics of HA in the synovium can contribute to inflammation. Decreased HA synthesis, increased HA degradation, and elevated oxidative stress all lead to a decrease in both concentration and average molecular weight of the HA present in the synovium.23,24 Multiple studies have demonstrated that exposure of chondrocytes and fibroblasts to LMW HA fragments (<400 kDa) can cause an upregulation of pro-inflammatory cytokines.39–41 In addition, it has been shown that the levels of IL-18 and IL-33 are increased in mouse synovial fibroblasts after exposure to HA fragments,40 and that HA fragments enhance the inflammatory activity of macrophages.42 Table 1 summarizes the large number of pro-inflammatory molecules whose genes are induced by HA fragments and the cell types in which this occurs.42 In contrast, HMW HA appears to have the opposite effect on some of these systems, suppressing mediators such as TNF-α and IL-1β.43–46
Table 1.
Selected genes that are induced by HA fragments and the cells in which this occurs.42
| Category | Gene/protein | Cell type |
|---|---|---|
| Chemokines | CCL3 | Macrophages |
| CCL4 | Macrophages | |
| CXCL2 | Macrophages | |
| CCL5 | Macrophages | |
| CCL2 | Renal tubular epithelial cells | |
| CXCL10 | Macrophage | |
| CXCL9 | Macrophage | |
| CXCL1 | Endothelial cells | |
| CCL5 | Macrophages | |
| IL-8 | Endothelial cells, epithelial cells | |
| CXCL1 | Macrophages | |
| Cytokines | IL-12 | Macrophages, dendritic cells |
| TNF-α | Dendritic cells | |
| IL-1β | Dendritic cells | |
| Growth factors | TGF-β2 | Monocytes |
| IGF-I | Macrophages | |
| Transcription factors | IκBα | Macrophages |
| AP-1 | Endothelial cells | |
| Rest | Monocytes | |
| ECM | MMP-10 | Endothelial cells |
| MMP-13 | Monocytes, dendritic cells | |
| PAI-1 | Macrophages | |
| uPA | Macrophages | |
| MME | Macrophages | |
| MMP-9 | Dendritic cells | |
| Collagen VIII | Endothelial cells | |
| HSPG | Syndecan-4 | Endothelial cells |
| Others | iNOS | Hepatocytes, endothelial, Kupffer, and stellate cells |
| COX-2 | Renal tubular epithelial cells | |
| MDR-1 | Lymphocytes | |
| Trdn | Monocytes | |
| Frk | Monocytes |
Abbreviations: AP-1, activator protein 1; CCL, chemokine ligand; COX-2, cyclooxygenase 2; CXC, chemokine receptor; ECM, extracellular matrix; Frk, fractalkine; HA, hyaluronan; HSPG, heparin sulfate proteoglycan; IGF-1, insulinlike growth factor 1; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor α; IL, interleukin; iNOS, inducible nitric oxide synthase; MDR-1, multidrug resistance protein 1; MME, macrophage metalloelastase; MMP, matrix metalloproteinase; PAI-1, plasminogen activator inhibitor 1; TGF-β2, transforming growth factor β2; TNF-α, tumor necrosis factor α; Trdn, triadin; uPA, urokinase-type plasminogen activator.
Hyaluronan injection for treatment of OA of the knee is regulated by the US Food and Drug Administration (FDA) as a medical device; however, HMW HA, such as that used in viscosupplementation, also has multiple effects on molecular signaling pathways in several cell types found in synovial joints and contributes to the homeostasis of synovial joints (Table 2). The HA receptor activity may be responsible for the prolonged pain relief effect with intra-articular HA therapy, even though the residence time of the exogenous molecule within the joint is quite short.54
Table 2.
Selected genes that are suppressed by HMW HA and the cells in which this occurs.
| Category | Gene/protein | Cell type | References |
|---|---|---|---|
| Cytokines | IFN-γ | Chondrocytes | Campo et al, 201043 |
| IL-1β | Chondrocytes | Chang et al, 201245 | |
| Macrophages | Baeva et al, 201446 | ||
| Synoviocytes | Wang et al, 200644 | ||
| IL-6 | Chondrocytes | Campo et al, 201043 | |
| Macrophages | Yasuda et al, 201147 | ||
| Synoviocytes | Wang et al, 200644 | ||
| LIF | Synoviocytes | Wan et al, 200644 | |
| RANKL | Osteoblasts | Ariyoshi et al, 201448 | |
| TNF-α | Chondrocytes | Campo et al, 201043 | |
| Synoviocytes | Wang et al, 200644 | ||
| Chemokines | CCL5 (RANTES) | Chondrocytes | Tanaka et al, 200649 |
| IL-8 | Synoviocytes | Wang et al, 200644 | |
| Transcription factors | Phospho-Akt/PKB | Macrophages | Yasuda et al, 201147 |
| NF-κB | Chondrocytes | Chang et al, 201245 | |
| Macrophages | Yasuda et al, 201047 | ||
| Phospho-JNK | Synoviocytes | Kataoka et al, 201350 | |
| Phospho-p38 MAPK | Chondrocytes | Yasuda, 201051 | |
| Synoviocytes | Kataoka et al, 201350 | ||
| Phospho-ERK | Chondrocytes | Hashizume et al, 201052 | |
| Proteases | ADAM17 (TACE) | Synoviocytes | Wang et al, 200644 |
| ADAMTS4 (aggrecanase-1) | Synoviocytes | Wang et al, 2006; Kataoka et al, 201344,50 | |
| ADAMTS5 (aggrecanase-2) | Synoviocytes | Wang et al, 200644 | |
| MMP-1 | Chondrocytes | Hashizume et al, 201052 | |
| Synoviocytes | Wang et al, 200644 | ||
| MMP-2 | Synoviocytes | Wang et al, 200644 | |
| MMP-3 | Chondrocytes | Hashizume et al, 201052 | |
| Synoviocytes | Wang et al, 200644 | ||
| MMP-9 | Synoviocytes | Wang et al, 200644 | |
| MMP-13 | Chondrocytes | Hashizume et al, 201052 | |
| Synoviocytes | Wang et al, 200644 | ||
| TIMP-1 | Synoviocytes | Wang et al, 200644 | |
| TIMP-2 | Synoviocytes | Wang et al, 200644 | |
| Others | COX-2 | Macrophages | Yasuda et al, 201051 |
| iNOS | Chondrocytes | Campo et al, 201043 | |
| Synoviocytes | Wang et al, 200644 | ||
| TLR-4 | Chondrocytes | Campo et al, 201053 |
Abbreviations: ADAM, a disintegrin and metalloprotease; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; Akt, Ak strain transforming; CCL, chemokine ligand; COX-2, cyclooxygenase 2; ERK, extracellular signal–related kinase; HA, hyaluronan; HMW, high molecular weight; IFN-γ, interferon γ; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-jun N-terminal kinase; LIF, leukemia inhibitory factor; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NF-κB, nuclear factor κB; PKB, protein kinase B; RANKL, receptor activator of nuclear factor κB ligand; RANTES, regulated on activation; normal T cell expressed and secreted; TACE, tumor necrosis factor α–converting enzyme; TIMP, tissue inhibitor of metalloproteinases; TLR-4, toll-like receptor 4; TNF-α, tumor necrosis factor α.
Antinociceptive and Anti-Inflammatory Actions of HA
Antinociceptive effects
Hyaluronan products have certain rheologic properties that inhibit joint nociceptor discharges by acting as an elastoviscous filter. There is also a response to chemical sensitization of nociceptive terminals of inflamed joint tissues, possibly linked to HA concentration.55 Results from a study in cats with experimental arthritis indicated that intra-articular injection of HMW HA reduced the activity of pain-related primary afferents at baseline and during movement,56 suggesting that joint lubrication is not solely responsible for the antinociceptive effects of HA. Hyaluronan may coat pain receptors in synovial tissues and perhaps also trap molecules involved in pain signaling.57 Recently, single intra-articular injections of HA were shown to decrease pain by more than 50% compared with saline in a bradykinin/prostaglandin E2 (PGE2) model.58 In addition, a single injection of HA-attenuated pain responses for at least 56 days after administration.58 Injection of HA into the superior compartment of the temporomandibular joint in patients with unilateral internal derangement has been shown to significantly decrease pain (P < .01), as well as joint levels of leukotriene C4, 6-keto-prostaglandin F1α, prostaglandin F2α, and IL-1β (all P’s < .05).59 Experiments using human macrophages have indicated that HMW HA interferes with lipopolysaccharide (LPS)-induced increases in the production of PGE2 and cyclooxygenase 2 (COX-2). In these cells, pretreatment with HA suppressed induction of COX-2, leading to a decrease in PGE2 production. Use of an anti-CD44 antibody reversed the inhibitory effects of HA on the LPS-mediated increase in PGE2 production and COX-2 induction, indicating that the anti-inflammatory effects of HA were CD44 receptor mediated.51
Additional mechanisms that may contribute to the antinociceptive effects of HA include an inhibition of arachidonic acid release from fibroblasts and activation of opioid receptors.60,61 Exposure to HA has been shown to decrease secretion of arachidonic acid from fibroblasts taken from patients with knee OA and stimulated with bradykinin or induced by a calcium ionophore.60 More recent in vitro experiments demonstrated that HA stimulated κ opioid receptors expressed by Chinese hamster ovary cells and rat dorsal root ganglion neurons.61
Results from one study have indicated that unlike anti-inflammatory drugs, pain reduction resulting from HA administration was associated with cartilage preservation. Both HA and loxoprofen significantly decreased pain in a rabbit OA model (partial meniscectomy), as measured by hind paw weight distribution, and they also reduced PGE2 production. Hyaluronan treatment also significantly inhibited cartilage degeneration, whereas loxoprofen did not.52
Anti-inflammatory effects
High-molecular-weight HA has the potential to inhibit the inflammatory events involved in OA by interfering with the actions of LMW HA fragments at CD44, RHAMM, and TLR-2 and TLR-4. Results from in vitro and in vivo studies indicate that administration of HMW HA has significant anti-inflammatory effects that are mediated, at least in part, by blockade of CD44. Administration of HMW HA leads to the downregulation of IL-8 and inducible NO synthase gene expression in cells that were not stimulated with IL-1. In cells that were stimulated with IL-1, TNF-α gene expression was also downregulated. Blocking CD44 with a specific antibody inhibited the effects of HMW HA on pro-inflammatory gene expression.44,62,63 It has also recently been shown that HMW HA suppresses IL-1β production in monocyte/macrophage cultures under various inflammatory conditions.46
Inhibition of IL-1β and TNF-α production by HA has important downstream effects on the expression of pro-inflammatory and catabolic molecules. IL-1β induces ADAMTS via p38 mitogen-activated protein kinase and c-jun NH2-terminal kinase phosphorylation in human fibroblast-like synoviocytes. The ADAMTS degrade aggrecan in cartilage; HMW HA also suppresses ADAMTS expression.50
IL-1β downregulates peroxisome proliferator–activated receptor γ (PPARγ) and increases expression of MMPs.45 Results from a study focused on inflammatory gene expression in IL-1β–stimulated human chondrosarcoma cells indicate that HMW HA increases the expression of PPARγ and decreases that for COX-2, MMP-1, and MMP-13. Additional anti-inflammatory effects of HA demonstrated in this study included suppression of mitogen-activated protein kinases and nuclear factor κB signaling.45
In a rabbit model that induced OA through sterile papain solution injection into the knee, an intra-articular injection of HA resulted in significant reductions in the expression of IL-1β and TNF-α and increased expression of TIMP-1 compared with intra-articular saline-treated controls.64 Histologic analysis and Mankin scores also indicated significantly less degeneration in the HA-treated vs control animals (P < .01). Treatment with HA also resulted in proliferation of chondrocytes in this model.64
Exogenous HA also decreases levels of inflammatory cytokines and MMPs in tissues taken from patients with OA and other conditions associated with joint damage. In one study, subacromial synovium fibroblasts were taken from patients with rotator cuff disease and stimulated with IL-1β. Addition of HA resulted in a dose-dependent decrease in the expression of IL-1β, TNF-α, and IL-6. These effects of HA were lost when CD44 was blocked with the anti-CD44 antibody, OS/37.65 Incubation with HA has also been shown to inhibit IL-1β–induced MMP activity in explants of synovial tissue from patients with OA. This effect was also potentially mediated by interaction of HA with CD44.66
Oxidative stress resulting from chronic overproduction of ROS plays an important role in OA, and this stress may be induced by abnormal cyclic loading of the joint.67,68 Free radicals can damage DNA, decrease cell viability, and contribute to disruption of the ECM. Reactive oxygen species reduce synthesis of proteoglycans and accelerate chondrocyte senescence and thus their ability to repair tissue.68
Results from several studies have demonstrated that HA reduces levels of ROS and also protects chondrocytes from the adverse consequences of exposure to these molecules. Several experiments have shown that HA can decrease ROS production resulting from mechanical stress. For example, mechanical compression of bovine cartilage increases ROS production, and this effect is attenuated by incubating the tissue with HA.69 In a rabbit model in which OA was induced by intra-articular injection of papain, treatment with HA significantly decreased the expression of NO vs control animals treated with saline (P < .05).64 It has also been shown that HA inhibits NO-induced apoptosis and dedifferentiation of chondrocytes in vitro.70
Increased expression of MMPs and aggrecanases contributes to the cartilage-destructive characteristic of OA,71 and intra-articular administration of HA has been shown to significantly inhibit these proteases. In human chondrocytes, stimulation with IL-6 and the IL-6 soluble receptor increases expression of MMP-1, MMP-3, and MMP-13. The effect is inhibited by HA via the CD44 receptor. In vitro incubation of human chondrocytes with an anti-CD44 antibody blocks this action, indicating that interaction of HA with CD44 is necessary for inhibition of MMP upregulation.72 In a rabbit model of OA induced by anterior cruciate ligament transection, intra-articular injection of HA decreased OA severity and suppressed expression of MMP-13 in subchondral bone. These effects also required interaction of HA with CD44.73 In a different rabbit OA model that induced disease by injection of papain, treatment with HA significantly increased synovial fluid levels of TIMP-1, an inhibitor of MMPs.64
Furthermore, HA has been shown to decrease MMP expression in patients with OA. In a trial of 51 patients with knee OA who received intra-articular injections of HA or chondroitin sulfate and were followed for 6 months, both treatments significantly decreased pain/inflammation scores (P < .01). However, only HA significantly reduced levels of MMP-9 (P < .01).74
Disease-Modifying Effects of OA—Tissue Protection
Exogenous HA—preservation and restoration of the ECM
During the progression of OA, cartilage ECM is remodeled by proteases expressed by chondrocytes in response to inflammation. Changes in the ECM alter the biomechanical environment of chondrocytes and result in disease progression.75 The ECM is integrally involved in the development and progression of OA, and its preservation and restoration have become the focal point of treatment.
Results from several studies have indicated that exogenous HA can increase the synthesis of ECM molecules.74,76 Exogenous HA stimulates synovial fibroblasts to produce new HA. When synovial fibroblasts from OA knees were cultured with HA formulations of various molecular weights, the amount of newly synthesized HA was dependent on both the concentration and molecular weight of the exogenous HA. Higher molecular weight agents stimulated more HA synthesis and very LMW HA suppressed HA synthesis when applied at high concentrations.25 Two additional studies have shown that intra-articular injection of HA in patients with OA increases endogenous HA production.74,76
In vitro experiments that treated bovine articular chondrocytes with HA induced a significant increase in sulfated glycosaminoglycan and hydroxyproline synthesis, which was coincident with increased matrix deposition of chondroitin-6-sulfate and collagen type II.77 Mechanical stress resulting in injury has been shown to result in loss of proteoglycans from cartilage and can play a role in the development and progression of OA,78 whereas administration of HA has been shown to increase proteoglycan synthesis in cartilage subjected to mechanical stress.69
Osteopontin is an extracellular scaffold protein that is upregulated in OA cartilage and inhibits IL-1β–induced NO and PGE2 production in human OA–affected cartilage in response to joint inflammation.79 It has been shown that exposure to HA significantly increases osteopontin expression in fibroblast-like synoviocytes from patients with OA of the knee, thus potentially amplifying its anti-inflammatory actions.80
Exogenous HA—clinical evidence of tissue protection
Although there is a host of basic science and animal studies that demonstrate the ability of HA to significantly blunt rheologic, inflammatory, and physical changes brought about by OA, there has been less focus on support of this potential clinically in human studies. In 2007, a review of disease-modifying OA drugs dedicated only a single paragraph to HA, concluding that there was no evidence that HA provided a disease-modifying effect.81 This conclusion was based on the observation that intra-articular HA injection into the knee had no significant effect on radiographic progression vs intra-articular saline over 1 year of follow-up.82 However, a subgroup analysis of patients with less severe disease at baseline indicated significantly less joint space narrowing with HA vs saline (P = .02).82 Ultimately, however, there is a growing consensus that plain film radiographs may be insensitive to potential disease-modifying effects of OA treatments that are currently used as well as those in development.83
As imaging technology becomes more advanced, the macroscopic impact of HA supplementation in both experimental animals and clinical patients will become more evident. For example, in a study that employed an anterior cruciate ligament transection model of OA in rats, T2-weighted magnetic resonance (MR) imaging was used to evaluate the effects of intra-articular injection of HA, intra-articular saline, and sham injections. Study results indicated significant superiority of intra-articular HA over intra-articular saline for T2 MR values (P < .05). Study results also showed that the T2 values were significantly and positively correlated with Mankin scores.84 Results from a study of patients evaluated with T1ρ and T2 MR imaging just prior to total knee arthroplasty indicated T1ρ mapping was superior to T2 mapping for evaluation of denatured articular cartilage associated with OA of the knee.85 A clinical trial using T1ρ MR imaging to evaluate effects of intra-articular HA injection is currently under way. Further human studies are needed to demonstrate that these basic science principles can indeed translate into disease modification in human OA pathology.
The importance of evaluating outcomes relevant to the patient, such as pain and quality of life, alongside imaging, cannot be overstated, as approximately two-thirds of adult patients with radiographic evidence of knee OA are asymptomatic.5 Since 2007, studies have investigated the potentially beneficial effects of HA in relieving OA pain and reducing tissue destruction.55 A 2006 Cochrane Review of randomized trials concluded that viscosupplementation with HA (or hylan derivatives) was superior to placebo in improving pain and function at several weeks. Furthermore, viscosupplementation generally demonstrated benefit for a longer duration compared with intra-articular corticosteroid injections.86 More recent systematic reviews have also shown safety and efficacy of HA over nonsteroidal anti-inflammatory drugs and other nonoperative treatment modalities.87,88
Conclusions
Hyaluronan products used for viscosupplementation are considered medical devices by the FDA. It has become increasingly apparent that HA influences a wide range of biologic processes via multiple molecular pathways (Figure 1). This review presents evidence for the broader role of HA in the treatment of OA beyond joint cushioning and lubrication. Exogenous HA can reduce pain transmission and blunt the inflammatory cascade via the CD44 receptor that is associated with OA, as well as stimulate synthesis and deposition of ECM molecules that are suppressed and degraded in an osteoarthritic joint. These data also show that the effect of HA is dependent on the size of the fragment. In particular, long-chain HMW HA exerts an anti-inflammatory effect and can stimulate the production of endogenous HA, whereas shorter HA fragments are pro-inflammatory and can inhibit HA production at high concentrations. Although there are many molecules that will reduce the pain and inflammation due to OA, HA has the potential to reduce pain as well as to protect and restore the chondral matrix.
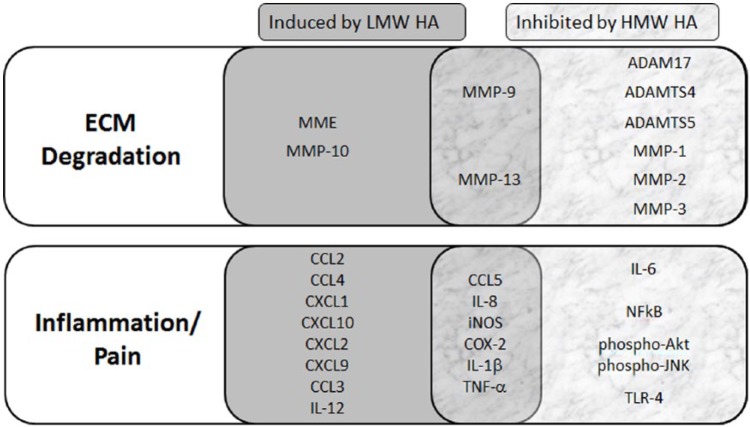
Figure 1.
Selected genes that are involved in ECM degradation (top) and inflammation/pain (bottom) responses in the OA environment. Data on protein function were obtained by searching the Universal Protein Resource (UniProt; www.uniprot.org) and analyzing the gene ontology associated with each protein. Genes within the gray (left) box are induced by LMW HA fragments and those within the marble (right) box are inhibited by HMW HA. The overlapping region (center) show selected genes that have been shown to be both induced by LMW HA and inhibited by HMW HA. ADAM indicates a disintegrin and metalloprotease; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; Akt, Ak strain transforming; CCL, chemokine ligand; COX-2, cyclooxygenase 2; CXC, chemokine receptor; ECM, extracellular matrix; HA, hyaluronan; HMW, high molecular weight; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-jun N-terminal kinase; LMW, low molecular weight; MME, macrophage metalloelastase; MMP, matrix metalloproteinase; NF-κB, nuclear factor κB; OA, osteoarthritis; TLR-4, toll-like receptor 4; TNF-α, tumor necrosis factor α.
Much of what is known regarding the biochemical actions of HA in the knee OA environment comes from experiments with animal models and human explants. Only a small number of clinical studies have evaluated the biochemical effects of HA in patients with OA. Large-scale clinical trials that evaluate biomarker changes in response to treatment, as well as noninvasive imaging studies, would be beneficial for further elucidating the mechanism(s) of action of HA in OA, demonstrating disease modification in vivo and providing insight into additional biological targets for treatment of this very common disease.
Acknowledgments
The authors would like to thank Global Research Solutions Inc. for their medical writing assistance.
Footnotes
Peer Review:Two peer reviewers contributed to the peer review report. Reviewers’ reports totaled 238 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Ferring Pharmaceuticals Inc.
Declaration of Conflicting Interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.A.N. serves on advisory boards for Ferring. A.F. and F.N. are paid employees of Ferring Pharmaceuticals Inc. M.B. is a consultant for Ferring, Smith & Nephew, Stryker, Amgen, Zimmer, Moximed, Bioventus, Merck, Eli Lilly, Sanofi, CONMED. Grants/grants pending from Stryker, Zimmer, Amgen, Smith & Nephew, DePuy, Eli Lilly, and Bioventus.
Author Contributions: MAN and MB were responsible for the development and writing of the manuscript. MAN, AF, FN, and MB provided critical review and revisions to the manuscript draft. All authors have read and approved this manuscript.
References
- 1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. [DOI] [PubMed] [Google Scholar]
- 2. Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112:S13–S19. [DOI] [PubMed] [Google Scholar]
- 3. Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dillon CF, Rasch EK, Gu Q , Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-1994. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 6. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lohmander LS, Dalén N, Englund G, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Ann Rheum Dis. 1996;55:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petrella RJ, Petrella M. A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol. 2006;33:951–956. [PubMed] [Google Scholar]
- 9. Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154–162. [DOI] [PubMed] [Google Scholar]
- 10. Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum. 2009;39:1–9. [DOI] [PubMed] [Google Scholar]
- 11. Navarro-Sarabia F, Coronel P, Collantes E, et al. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011;70:1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh P. The role of hyaluronic acid (hyaluronan) in health and disease: interactions with cells, cartilage and components of synovial fluid. Clin Exp Rheumatol. 1994;12:75–82. [PubMed] [Google Scholar]
- 13. Young DA, Bui C, Barter MJ. Understanding CpG methylation in the context of osteoarthritis. Epigenomics. 2012;4:593–595. [DOI] [PubMed] [Google Scholar]
- 14. Chen LJ, Wei SY, Chiu JJ. Mechanical regulation of epigenetics in vascular biology and pathobiology. J Cell Mol Med. 2013;17:437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bui C, Barter MJ, Scott JL, et al. cAMP response element-binding (CREB) recruitment following a specific CpG demethylation leads to the elevated expression of the matrix metalloproteinase 13 in human articular chondrocytes and osteoarthritis. FASEB J. 2012;26:3000–3011. [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto K, Otero M, Imagawa K, et al. Regulated transcription of human matrix metalloproteinase 13 (MMP13) and interleukin-1β (IL1B) genes in chondrocytes depends on methylation of specific proximal promoter CpG sites. J Biol Chem. 2013;288:10061–10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abd-Allah SH, Shalaby SM, Pasha HF, El-Shal AS, Abou El-Saoud AM. Variation of matrix metalloproteinase 1 and 3 haplotypes and their serum levels in patients with rheumatoid arthritis and osteoarthritis. Genet Test Mol Biomarkers. 2012;16:15–20. [DOI] [PubMed] [Google Scholar]
- 19. Kerna I, Kisand K, Tamm AE, Lintrop M, Veske K, Tamm AO. Missense single nucleotide polymorphism of the ADAM12 gene is associated with radiographic knee osteoarthritis in middle-aged Estonian cohort. Osteoarthritis Cartilage. 2009;17:1093–1098. [DOI] [PubMed] [Google Scholar]
- 20. Roach HI, Yamada N, Cheung KS, et al. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005;52:3110–3124. [DOI] [PubMed] [Google Scholar]
- 21. Reynard LN, Loughlin J. Genetics and epigenetics of osteoarthritis. Maturitas. 2012;71:200–204. [DOI] [PubMed] [Google Scholar]
- 22. Goldring MB, Marcu KB. Epigenomic and microRNA-mediated regulation in cartilage development, homeostasis, and osteoarthritis. Trends Mol Med. 2012;18:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921–1943. [DOI] [PubMed] [Google Scholar]
- 24. Yamazaki K, Fukuda K, Matsukawa M, et al. Reactive oxygen species depolymerize hyaluronan: involvement of the hydroxyl radical. Pathophysiology. 2003;9:215–220. [DOI] [PubMed] [Google Scholar]
- 25. Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987;7:113–122. [DOI] [PubMed] [Google Scholar]
- 26. Prasadam I, Crawford R, Xiao Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes—possible pathogenic role in osteoarthritis. J Rheumatol. 2012;39:621–634. [DOI] [PubMed] [Google Scholar]
- 27. Radwan M, Gavriilidis C, Robinson JH, et al. Matrix metalloproteinase 13 expression in response to double-stranded RNA in human chondrocytes. Arthritis Rheum. 2013;65:1290–1301. [DOI] [PubMed] [Google Scholar]
- 28. Li NG, Shi ZH, Tang YP, et al. New hope for the treatment of osteoarthritis through selective inhibition of MMP-13. Curr Med Chem. 2011;18:977–1001. [DOI] [PubMed] [Google Scholar]
- 29. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- 30. Dunn S, Kolomytkin OV, Waddell DD, Marino AA. Hyaluronan-binding receptors: possible involvement in osteoarthritis. Mod Rheumatol. 2009;19:151–155. [DOI] [PubMed] [Google Scholar]
- 31. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santini P, Politi L, Vedova PD, Scandurra R, Scotto d’Abusco A. The inflammatory circuitry of miR-149 as a pathological mechanism in osteoarthritis. Rheumatol Int. 2013;34:711–716. [DOI] [PubMed] [Google Scholar]
- 33. Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. [DOI] [PubMed] [Google Scholar]
- 34. McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31:1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beekhuizen M, Gierman LM, van Spil WE, et al. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis Cartilage. 2013;21:918–922. [DOI] [PubMed] [Google Scholar]
- 36. Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. [DOI] [PubMed] [Google Scholar]
- 37. Greene GW, Banquy X, Lee DW, Lowrey DD, Yu J, Israelachvili JN. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci U S A. 2011;108:5255–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swann DA, Radin EL, Nazimiec M, Weisser PA, Curran N, Lewinnek G. Role of hyaluronic acid in joint lubrication. Ann Rheum Dis. 1974;33:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campo GM, Avenoso A, D’Ascola A, et al. Adenosine A2A receptor activation and hyaluronan fragment inhibition reduce inflammation in mouse articular chondrocytes stimulated with interleukin-1β. FEBS J. 2012;279:2120–2133. [DOI] [PubMed] [Google Scholar]
- 40. Campo GM, Avenoso A, D’Ascola A, et al. 6-Mer hyaluronan oligosaccharides increase IL-18 and IL-33 production in mouse synovial fibroblasts subjected to collagen-induced arthritis. Innate Immun. 2012;18:675–684. [DOI] [PubMed] [Google Scholar]
- 41. Campo GM, Avenoso A, D’Ascola A, et al. The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem. 2012;113:1852–1867. [DOI] [PubMed] [Google Scholar]
- 42. Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campo GM, Avenoso A, Campo S, et al. Differential effect of molecular mass hyaluronan on lipopolysaccharide-induced damage in chondrocytes. Innate Immun. 2010;16:48–63. [DOI] [PubMed] [Google Scholar]
- 44. Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247. [DOI] [PubMed] [Google Scholar]
- 45. Chang CC, Hsieh MS, Liao ST, et al. Hyaluronan regulates PPARγ and inflammatory responses in IL-1β-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr Polym. 2012;90:1168–1175. [DOI] [PubMed] [Google Scholar]
- 46. Baeva LF, Lyle DB, Rios M, Langone JJ, Lightfoote MM. Different molecular weight hyaluronic acid effects on human macrophage interleukin 1β production. J Biomed Mater Res A. 2014;102:305–314. [DOI] [PubMed] [Google Scholar]
- 47. Yasuda T. Hyaluronan inhibits Akt, leading to nuclear factor-κB down-regulation in lipopolysaccharide-stimulated U937 macrophages. J Pharmacol Sci. 2011;115:509–515. [DOI] [PubMed] [Google Scholar]
- 48. Ariyoshi W, Okinaga T, Knudson CB, Nishiara T. High molecular weight hyaluronic acid regulates osteoclast formation by inhibiting receptor activator of NF-κB ligand through Rho kinase. Osteoarthritis Cartilage. 2014;22:111–120. [DOI] [PubMed] [Google Scholar]
- 49. Tanaka M, Masuko-Hongo K, Kato T, Nishioka K, Nakamura H. Suppressive effects of hyaluronan on MMP-1 and RANTES production from chondrocytes. Rheumatol Int. 2006;26:185–190. [DOI] [PubMed] [Google Scholar]
- 50. Kataoka Y, Ariyoshi W, Okinaga T, et al. Mechanisms involved in suppression of ADAMTS4 expression in synoviocytes by high molecular weight hyaluronic acid. Biochem Biophys Res Commun. 2013;432:580–585. [DOI] [PubMed] [Google Scholar]
- 51. Yasuda T. Hyaluronan inhibits prostaglandin E2 production via CD44 in U937 human macrophages. Tohoku J Exp Med. 2010;220:229–235. [DOI] [PubMed] [Google Scholar]
- 52. Hashizume M, Koike N, Yoshida H, Suzuki M, Mihara M. High molecular weight hyaluronic acid relieved joint pain and prevented the progression of cartilage degeneration in a rabbit osteoarthritis model after onset of arthritis. Mod Rheumatol. 2010;20:432–438. [DOI] [PubMed] [Google Scholar]
- 53. Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie. 2010;92:204–215. [DOI] [PubMed] [Google Scholar]
- 54. Brandt KD, Smith GN, Jr, Simon LS. Intraarticular injection of hylaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000;43:1192–1203. [DOI] [PubMed] [Google Scholar]
- 55. Gomis A, Miralles A, Schmidt RF, Belmonte C. Intra-articular injections of hyaluronan solutions of different elastoviscosity reduce nociceptive nerve activity in a model of osteoarthritic knee joint of the guinea pig. Osteoarthritis Cartilage. 2009;17:798–804. [DOI] [PubMed] [Google Scholar]
- 56. Pozo MA, Balazs EA, Belmonte C. Reduction of sensory responses to passive movements of inflamed knee joints by hylan, a hyaluronan derivative. Exp Brain Res. 1997;116:3–9. [DOI] [PubMed] [Google Scholar]
- 57. Gotoh S, Miyazaki K, Onaya J, Sakamoto T, Tokuyasu K, Namiki O. [Experimental knee pain model in rats and analgesic effect of sodium hyaluronate (SPH)]. Nihon Yakurigaku Zasshi. 1988;92:17–27. [DOI] [PubMed] [Google Scholar]
- 58. Boettger MK, Kümmel D, Harrison A, Schaible HG. Evaluation of long-term antinociceptive properties of stabilized hyaluronic acid preparation (NASHA) in an animal model of repetitive joint pain. Arthritis Res Ther. 2011;13:R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hirota W. Intra-articular injection of hyaluronic acid reduces total amounts of leukotriene C4, 6-keto-prostaglandin F1alpha, prostaglandin F2alpha and interleukin-1beta in synovial fluid of patients with internal derangement in disorders of the temporomandibular joint. Br J Oral Maxillofac Surg. 1998;36:35–38. [DOI] [PubMed] [Google Scholar]
- 60. Tobetto K, Yasui T, Ando T, et al. Inhibitory effects of hyaluronan on [14C]arachidonic acid release from labeled human synovial fibroblasts. Jpn J Pharmacol. 1992;60:79–84. [DOI] [PubMed] [Google Scholar]
- 61. Zavan B, Ferroni L, Giorgi C, et al. Hyaluronic acid induces activation of the κ-opioid receptor. PLoS ONE. 2013;8:e55510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chou LW, Wang J, Chang PL, Hsieh YL. Hyaluronan modulates accumulation of hypoxia-inducible factor-1 alpha, inducible nitric oxide synthase, and matrix metalloproteinase-3 in the synovium of rat adjuvant-induced arthritis model. Arthritis Res Ther. 2011;13:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Campo GM, Avenoso A, Nastasi G, et al. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim Biophys Acta. 2011;1812:1170–1181. [DOI] [PubMed] [Google Scholar]
- 64. Chen L, Ling PX, Jin Y, Zhang TM. Hyaluronic acid in combination with chondroitin sulfate and hyaluronic acid improved the degeneration of synovium and cartilage equally in rabbits with osteoarthritis. Drug Discov Ther. 2011;5:190–194. [DOI] [PubMed] [Google Scholar]
- 65. Mitsui Y, Gotoh M, Nakama K, Yamada T, Higuchi F, Nagata K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J Orthop Res. 2008;26:1032–1037. [DOI] [PubMed] [Google Scholar]
- 66. Waddell DD, Kolomytkin OV, Dunn S, Marino AA. Hyaluronan suppresses IL-1beta-induced metalloproteinase activity from synovial tissue. Clin Orthop Relat Res. 2007;465:241–248. [DOI] [PubMed] [Google Scholar]
- 67. Beecher BR, Martin JA, Pedersen DR, Heiner AD, Buckwalter JA. Antioxidants block cyclic loading induced chondrocyte death. Iowa Orthop J. 2007;27:1–8. [PMC free article] [PubMed] [Google Scholar]
- 68. Heijink A, Gomoll AH, Madry H, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Miki Y, Teramura T, Tomiyama T, et al. Hyaluronan reversed proteoglycan synthesis inhibited by mechanical stress: possible involvement of antioxidant effect. Inflamm Res. 2010;59:471–477. [DOI] [PubMed] [Google Scholar]
- 70. Peng H, Zhou JL, Liu SQ, Hu QJ, Ming JH, Qiu B. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res. 2010;59:519–530. [DOI] [PubMed] [Google Scholar]
- 71. Pickarski M, Hayami T, Zhuo Y, Duong le T. Molecular changes in articular cartilage and subchondral bone in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. BMC Musculoskelet Disord. 2011;12:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hashizume M, Mihara M. High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem Biophys Res Commun. 2010;403:184–189. [DOI] [PubMed] [Google Scholar]
- 73. Hiraoka N, Takahashi KA, Arai Y, et al. Intra-articular injection of hyaluronan restores the aberrant expression of matrix metalloproteinase-13 in osteoarthritic subchondral bone. J Orthop Res. 2011;29:354–360. [DOI] [PubMed] [Google Scholar]
- 74. Shimizu M, Higuchi H, Takagishi K, Shinozaki T, Kobayashi T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15:51–56. [DOI] [PubMed] [Google Scholar]
- 75. Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bagga H, Burkhardt D, Sambrook P. March L. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946–950. [PubMed] [Google Scholar]
- 77. Akmal M, Singh A, Anand A, et al. The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg Br. 2005;87:1143–1149. [DOI] [PubMed] [Google Scholar]
- 78. Patwari P, Cheng DM, Cole AA, Kuettner KE, Grodzinsky AJ. Analysis of the relationship between peak stress and proteoglycan loss following injurious compression of human post-mortem knee and ankle cartilage. Biomech Model Mechanobiol. 2007;6:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Attur MG, Dave MN, Stuchin S, et al. Osteopontin: an intrinsic inhibitor of inflammation in cartilage. Arthritis Rheum. 2001;44:578–584. [DOI] [PubMed] [Google Scholar]
- 80. Zhang FJ, Gao SG, Cheng L, et al. The effect of hyaluronic acid on osteopontin and CD44 mRNA of fibroblast-like synoviocytes in patients with osteoarthritis of the knee. Rheumatol Int. 2013;33:79–83. [DOI] [PubMed] [Google Scholar]
- 81. Pelletier JP, Martel-Pelletier J. DMOAD developments: present and future. Bull NYU Hosp Jt Dis. 2007;65:242–248. [PubMed] [Google Scholar]
- 82. Jubb RW, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract. 2003;57:467–474. [PubMed] [Google Scholar]
- 83. Wang Y, Wluka AE, Jones G, Ding C, Cicuttini FM. Use magnetic resonance imaging to assess articular cartilage. Ther Adv Musculoskelet Dis. 2012;4:77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Huang GS, Lee HS, Chou MC, et al. Quantitative MR T2 measurement of articular cartilage to assess the treatment effect of intra-articular hyaluronic acid injection on experimental osteoarthritis induced by ACLX. Osteoarthritis Cartilage. 2010;18:54–60. [DOI] [PubMed] [Google Scholar]
- 85. Takayama Y, Hatakenaka M, Tsushima H, et al. T1ρ is superior to T2 mapping for the evaluation of articular cartilage denaturalization with osteoarthritis: radiological-pathological correlation after total knee arthroplasty. Eur J Radiol. 2013;82:e192–e198. [DOI] [PubMed] [Google Scholar]
- 86. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and meta-analysis. Ann Intern Med. 2015;162:46–54. [DOI] [PubMed] [Google Scholar]
- 88. Campbell KA, Erickson BJ, Saltzman BM, et al. Is local viscosupplementation injection clinically superior to other therapies in the treatment of osteoarthritis of the knee: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2036–2045. [DOI] [PubMed] [Google Scholar]