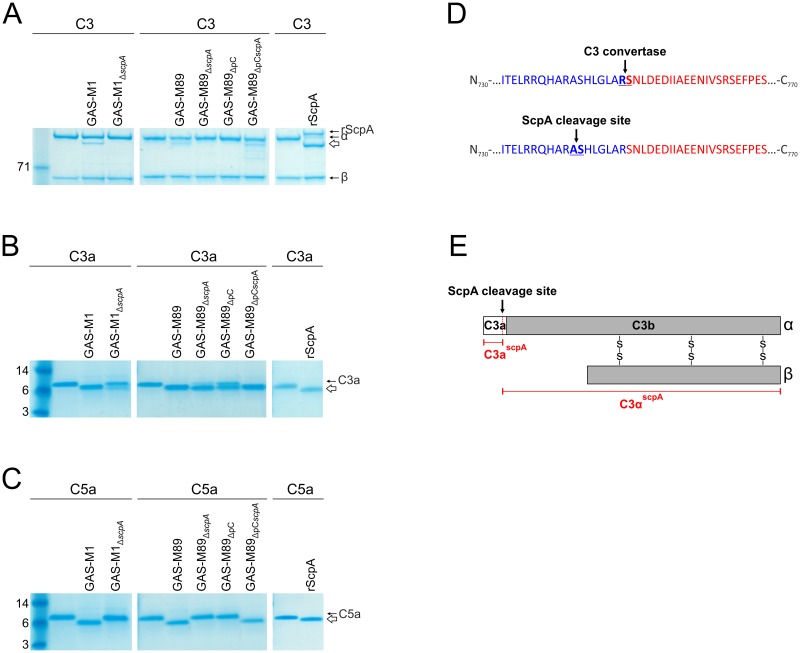
Fig 2. ScpA is necessary and sufficient for cleavage of human C3.
A-C) Cleavage of human complement factors A) C3, B) C3a and C) C5a by whole cell pellets (4x106 cfu) of GAS-M1 vs GAS-M1ΔscpA, GAS-M89 vs GAS-M89ΔscpA and rScpA (100 ng). Only bacterial strains expressing ScpA were able to cleave these complement factors. Cleaved moieties (C3αscpA, C3ascpA, C5ascpA) are indicated by a white arrow in each panel. D) C3αscpA was subjected to N-terminal sequencing. ScpA was demonstrated to cleave C3 between Ala741 and Ser742, 7aa N-terminal to the physiological C3 convertase cleavage site. E) Schematic representation of C3 α and β chains and the internal ScpA cleavage site. C3 is cleaved by C3 convertase to release C3b (grey bar) and C3a (white bar). The vertical black line between them shows the physiological C3 convertase cleavage site. ScpA cleaves C3 at an alternative site (red dotted line, black arrow) to release unique cleavage products, C3ascpA and C3αscpA (red text).