Abstract
Background
Enhanced platelet-derived growth factor receptor α (PDGFRα) signaling pathway activity leads to cardiac fibrosis. However, because of the pleiotropic effects of PDGFR signaling, its role in mediating the cardiac fibrotic response remains poorly understood. This study aimed to investigate the regulatory effect of c-Kit in cardiac fibroblasts activated by PDGFRα signaling.
Material/Methods
A cardiac fibrosis mice model was induced using isoproterenol, and the heart tissues of mice were tested through western blotting and real-time quantitative PCR (RT-qPCR). The cardiac fibroblasts of neonatal mice were treated with PDGF-AA or transfected with small interfering RNAs (siRNAs) specific for the mouse c-Kit gene. The levels of collagen I, collagen III, and alpha-smooth muscle actin (α-SMA) were analyzed using western blotting and RT-qPCR.
Results
In the heart of the cardiac fibrosis mice model, the activity of c-Kit was enhanced. PDGF-AA treatment accelerated the activity of c-Kit in cardiac fibroblasts. In addition, imatinib inhibited the activity of c-Kit in vivo and in vitro. Moreover, inhibition of c-Kit by siRNAs reduced the expression of α-SMA and collagens in the activated cardiac fibroblasts. Furthermore, PDGFRα directly bound c-Kit in cardiac fibroblasts and stimulated the expression of stem cell factor (SCF).
Conclusions
Our data demonstrated that PDGF/PDGFRα induced the activation of cardiac fibroblasts by activating c-Kit. This study indicated that c-Kit could be used as a potential therapeutic target for treatment of cardiac fibrosis.
MeSH Keywords: Fibroblasts; Proto-Oncogene Proteins c-Kit; Receptor, Platelet-Derived Growth Factor Alpha; Ventricular Remodeling
Background
Cardiac fibrosis serves an important role in the pathophysiology of cardiac disease and is correlated with elevated mortality in many heart diseases, such as sudden cardiac death, heart failure, and arrhythmia [1–3]. A key component of cardiac fibrosis is excessive extracellular matrix deposits in myocardial tissue [1]. Growing evidence shows that cardiac fibroblasts play the pivotal role in the occurrence, development, and outcome of cardiac fibrosis [3–5]. PDGFs and their receptors (PDGFRs) are known to stimulate the activation and differentiation of fibroblasts into myofibroblasts, which possess abundant protein α-SMA and express massive protein collagens [3,6,7]. Four PDGF ligands (PDGF-A, -B, -C, and -D) form five dimers (AA, BB, CC, DD, and AB) that bind to and activate two different tyrosine kinase receptors (PDGFRα and β) with different affinities [2]. PDGF-D/PDGFRβ activates the cardiac fibroblasts through activation of the TGF-β1 pathway [6]. Activated PDGFRα stimulates fibroblast proliferation by activating the AKT pathway [8]. Although there are many studies focusing on the role of the PDGF/PDGFR pathway in cardiac fibroblasts [2,3,6–8], the definite mechanisms responsible for PDGF/PDGFR signaling in activation of cardiac fibroblasts has not been uncovered.
Cellular Kit (c-Kit), also known as SCF receptor or CD117, is a member of the PDGFR family receptors (also called type-III receptor tyrosine kinase family), which includes PDGFRs, FMS-like tyrosine kinase 3 (FLT3), and macrophage colony stimulating factor 1 receptor (CSF-1R) [9]. After binding SCF, c-Kit is dimerized and activated by auto-phosphorylation of tyrosine residues in its intracellular domain [10]. Activated c-Kit can activate various downstream signaling pathways, including PI3K, MAPK, and JAK/STAT [10–12]. The c-Kit signaling pathway play a critical role in regulating survival, proliferation, differentiation, apoptosis, motility, and migration of c-Kit bearing cells [11,13–16]. During the development of the heart, c-Kit is transiently expressed in cardiomyocyte precursors [17]. Recently, c-Kit has been widely used as a surface antigen for identification and isolation of resident cardiac stem/progenitor cells (CPC) in the normal adult heart [18–20]. And c-Kit plays a crucial role in the growth and migration of human CPCs [13]. In addition, some reports showed that c-Kit was also expressed in fibroblasts from the heart [17,21]. Little is known, however, about c-Kit functions in the activation of cardiac fibroblasts.
In this study, we hypothesized that c-Kit plays an important role in regulating the occurrence of cardiac fibrosis and the activation of cardiac fibroblasts.
Material and Methods
Mice treatment
A cardiac fibrosis mice model was established and treated as described previously [22]. Briefly, C57BL/6 mice (10–12 weeks old, male) were randomly divided into four groups: vehicle (PBS), isoproterenol (ISO) (20 mg/kg, subcutaneous injection daily) (Sigma-Aldrich, USA), imatinib (40 mg/kg, intraperitoneal injection daily) (MedChem Express, USA), and ISO + imatinib for one week. After the aforementioned treatment, the mice were euthanized and the hearts were excised for biochemical analysis. Institutional Review Board approval was obtained from the Institutional Biosafety Committee of The First Affiliated Hospital, Sun Yat-sen University. All animal experiments adhered to the protocols approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University.
Cardiac fibroblasts isolation
Cardiac fibroblasts were harvested from neonatal C57BL/6 mice (2–4 days old) and cultured as reported previously [23,24].
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from the ventricle of the heart cells using TRIzol (Invitrogen, USA). Then 0.5 ug of total RNA was reverse-transcribed using TOYOBO ReverTra Ace kit (TOYOBO, Japan) according to the manufacturer’s protocols. The primers sequences as follows:
c-Kit, forward, 5′-GCAGGCTCATCCGCTCTGTAGT-3′,
reverse, 5′-GGCATGTTCTTCCACTGGTCCAC-3′.
Collagen I: forward, 5′-ACGTCCTGGTGAAGTTGGTC-3′, reverse,
5′-CAGGGAAGCCTCTTTCTCCT-3′.
Collagen III: forward, 5′-TGGCCCTGACCCAACTATGAT-3′,
reverse, 5′-GCACTTTTTGCCCTTCTTAATGTT-3′.
SCF, forward, 5′-GCACAGTGGCTGGTAACAGTTCAT-3′,
reverse, 5′-TACAGTGGCTGATGCTACGGAGTT-3′.
GAPDH, forward, 5′-CTCCTCCAAGGTCATCCATGACAACT-3′,
reverse, 5′-AACAAAGGGGCCATCCACAGTCTT-3′.
Quantitative PCR was performed in a LightCycle PCR system (Roche, Switzerland) by KOD SYBR qPCR Mix (TOYOBO) according to manufacturer’s protocol. GAPDH acted as internal standard. Relative quantification of expression was performed with 2−ΔΔCT method.
Western blotting
Total proteins were lysed by RIPA buffer (Beyotime, China). After centrifugation (4°C, 12,000 g for five minutes), protein concentrations were measured by Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, CA, USA). SDS-PAGE was conducted to separate equal amounts of proteins, which were blotted onto PVDF membrane (Millipore, USA). After blocked with milk, the membranes were incubated overnight at 4°C with primary antibodies. Primary antibodies were as follows: rabbit anti-p-c-Kit (Tyr719) antibody, rabbit anti-c-Kit antibody (CST, USA); rabbit anti-α-SMA antibody, rabbit anti-p-PDGFRα (Tyr720) antibody, rabbit anti-PDGFRα antibody (Abcam, USA); and mouse anti-GAPDH antibody (Proteintech, USA). Afterwards, membranes were incubated with the second antibody (Invitrogen, USA) at 25 °C for one hour. Finally, the blots were detected with Kodak film developer (Fujifilm, Japan). The protein blots were analyzed by the ImageJ software.
Cell transfection
The small interfering RNAs (siRNAs) used for cell transfection were synthesized by RiboBio Co., Ltd. (Guangzhou, China). The sequences were as follows: c-Kit, si-Kit1, 5′-CCGUGACAUUCAACGUUUATT-3′; si-Kit2, 5′-CUGUCUAGA AUUUACUCAATT-3′; negative control siRNA (si-NC), 5′-UUCUC CGAACGUGUCACGUTT-3′. All the siRNAs were transfected into cardiac fibroblasts using Lipofectamine 2000 Reagent (Life Technologies, Gaithersburg, MD, USA) according to the manufacturer’s protocol. After 72 hours, the cells were harvested for testing the interfering effect by western blotting and RT-qPCR.
Co-immunoprecipitation (Co-IP) assay
Cardiac fibroblasts were seeded into 100 mm culture dish and treated with PDGF-AA when cells reached 80–90%. Then, after 24 hour culture, the cells were lysed with lysis buffer at 4°C. The supernatants were collected by centrifugation at 4°C and quantified by the BCA assay. Briefly, 5 μg of rabbit anti-PDGFRα antibody was added into 500 μL (1 μg/μL) supernatants and then incubated overnight at 4°C. Then 60 μL protein A/G agarose beads (Thermo Fisher Scientific) were added to the antigen-antibody supernatants and incubation for two hours at 4°C. Afterwards, the cells were washed completely, and the co-immunoprecipitation (co-IP) product was obtained with 80 μL SDS-PAGE loading buffer. After incubation for five minutes at 100 °C, 40 μL of the co-IP products was used to perform western blotting analysis using anti-c-Kit antibody to validate the interaction between c-Kit and PDGFRα.
Statistical analysis
The statistical analysis of the data was performed using GraphPad Prism (Version 6.0, USA) and SPSS (Version 16.0, USA). ANOVA with Tukey’s multiple comparisons test was used for multiple comparisons and the Student’s t-test was performed to compare the differences in the means of two experiment groups. A p value of <0.05 was considered to be statistically significant.
Results
C-Kit activity is enhanced in cardiac fibrosis model
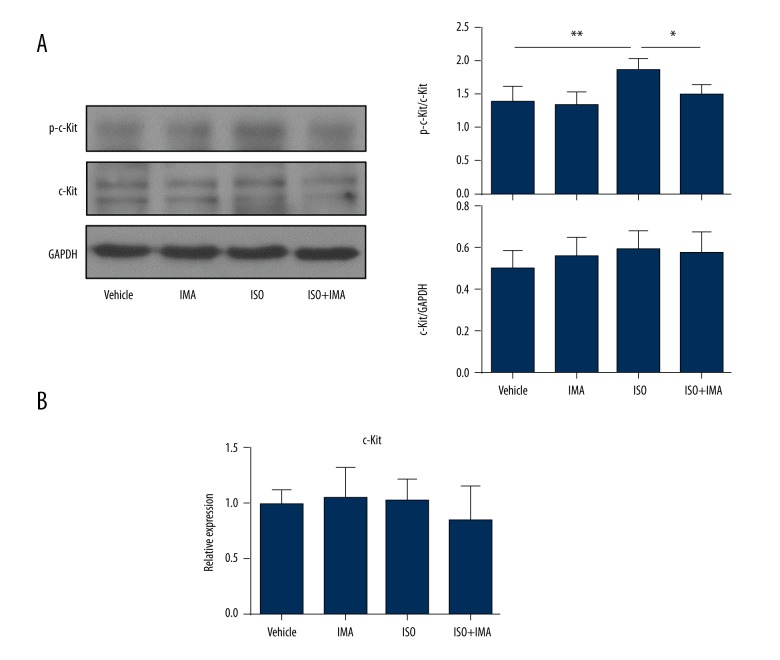
In our previous study [22], we successfully constructed a mice model of cardiac fibrosis using ISO. To assess the activity changes of c-Kit in a cardiac fibrosis model, we tested the phosphorylation state of c-Kit (p-Kit: Tyr719) in the hearts of cardiac fibrosis model mice by western blotting. As shown in Figure 1A, compared with that in the vehicle group, the myocardial phosphorylation form of c-Kit was significantly increased in the ISO treated group (p<0.01). Interestingly, ISO + imatinib treatment significantly inhibited c-Kit activity compared to ISO treatment only. However, the total protein level (Figure 1A) and the mRNA expression (Figure 1B) of c-Kit were not significant changed among the four groups.
Figure 1.
The kinase activity of c-Kit is enhanced in an animal model of cardiac fibrosis. (A) The lysates of heart tissues from a mice model treated with vehicle, imatinib (IMA), ISO, or imatinib + ISO for one week were analyzed for phosphorylation level of p-c-Kit (Tyr719) and total protein level of c-Kit. The western blotting results from one mouse in each group and the statistical analysis of the western blotting bands are shown. (B) The mRNA levels of c-Kit in hearts from four groups were tested by RT-qPCR. (n=5 for the vehicle group; n=8 for the imatinib group, the ISO group and the imatinib +ISO group). Data are expressed as mean ±SD. * Indicated p<0.05, and ** indicated p<0.01.
C-Kit kinase is activated in cardiac fibroblasts treated by PDGF-AA
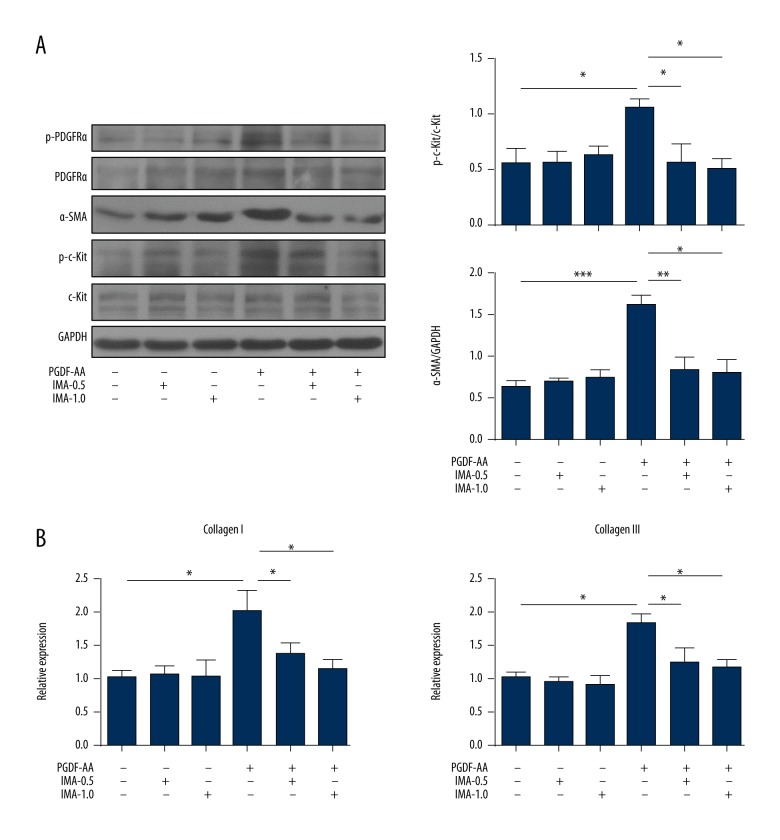
In our previous study [22], we found that the PDGF-A expression was significantly increased in the ISO group model heart, and the kinase activity of PDGFRα also was enhanced in the heart of the cardiac fibrosis model. PDGF-AA (activated form of PDGF-A) treatment could activate the fibroblasts by binding and activating PDGFRα [25]. These results indicated that PDGF-AA/PDGFRα was an important signaling pathway in the development of cardiac fibrosis induced by ISO. Next, we treated the primary cardiac fibroblasts with PDGF-AA (10 ng/mL) to explore the activity changes of c-Kit.
As shown in Figure 2, the expressions of α-SMA (Figure 2A) and collagens (Figure 2B) were significantly increased in the cardiac fibroblasts treated with PDGF-AA. Interestingly, PDGF-AA significantly increased the c-Kit activity in cardiac fibroblasts (Figure 2A) (p<0.05). However, the total protein of c-Kit was not significant changed in activated cardiac fibroblasts compared to that in untreated cells (Figure 2A). Moreover, the activation of PDGFRα and the phosphorylation of c-Kit were decreased in PDGF-AA + imatinib treatment compared to PDGF-AA treatment (Figure 2A).
Figure 2.
C-Kit kinase is activated in PDGF-AA-treated cardiac fibroblasts. (A) Mice cardiac fibroblasts were treated with PDGF-AA (10 ng/mL), imatinib (IMA)-0.5 (0.5 μM), IMA1.0 (1.0 μM), PDGF-AA + IMA-0.5, and PDGF-AA + IMA-1.0 for 24 hours. The lysates were analyzed for the expression of p-PDGFRα, PDGFRα, p-c-Kit, c-Kit, and α-SMA; quantitative analyses of p-c-Kit and α-SMA protein level are shown. (B) The mRNA expressions of collagen I and III were tested by RT-qPCR. The data are representative of three independent experiments. Data are expressed as mean ±SD. * Indicated p<0.05, ** indicated p<0.01, and *** indicated p<0.001.
Inhibition c-Kit by siRNA reduces the activation of cardiac fibroblasts
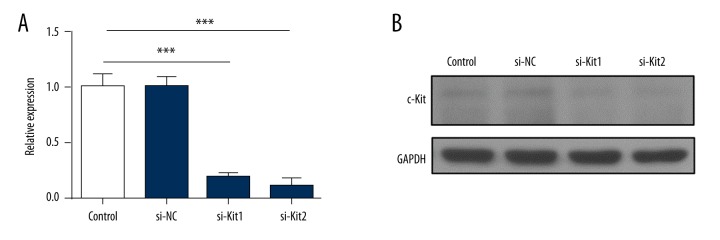
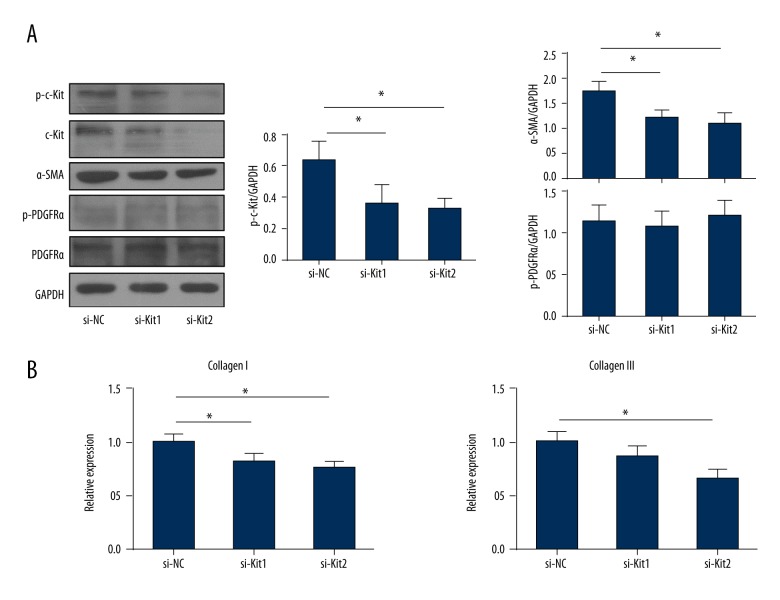
Imatinib inhibits not only the activation of PDGFRs kinases but also the kinase activity of c-Kit [26]. To further assess the role of c-Kit in the activation of cardiac fibroblasts, we utilized the siRNAs to inhibit the expression and the activity of c-Kit. As shown in Figure 3A and 3B, siRNAs (si-Kit1 and si-Kit2) significantly inhibited the mRNA level and the protein expression of c-Kit. After 48 hours of transfection with siRNAs, cardiac fibroblasts were treated with PDGF-AA and continually cultured for 24 hours. The kinase activity of c-Kit was significantly decreased in cardiac fibroblasts transfected with si-Kit1 or si-Kit2 when compared with that in cells transfected with si-NC (Figure 4A) (p<0.05). Inhibition c-Kit by siRNAs significantly reduced the expressions of α-SMA (Figure 4A) and collagens (Figure 4B) (p<0.05). However, the activity form and the total protein of PDGFRα were not significant changed among the three groups (Figure 4A). These data indicated that c-Kit played an important role in activation of cardiac fibroblasts and was a downstream factor of the PDGFRα signaling pathway.
Figure 3.
Inhibition effect of c-Kit by siRNAs. (A) Cardiac fibroblasts were transfected with si-NC, si-Kit1, and si-Kit2 and cultured for 72 hours. The mRNA expression of c-Kit was tested by RT-qPCR. (B) The lysates of transfected cardiac fibroblasts were analyzed by western blotting for the expression of c-Kit. The data are representative of three independent experiments. Data are expressed as mean ±SD. *** Indicated p<0.001.
Figure 4.
Inhibition c-Kit by siRNA reduces the expression of fibrosis related genes in activated cardiac fibroblasts. (A) After 48 hours of transfection with si-NC, si-Kit1, and si-Kit2, cardiac fibroblasts were treated with PDGF-AA (10 ng/mL) and continually cultured for 24 hours. The lysates were tested for the expression of p-c-Kit, c-Kit, p-PDGFRα, PDGFRα, and α-SMA; quantitative analyses of western blotting bands are shown. (B) The mRNA expressions of collagen I and III in transfected cardiac fibroblasts were tested by RT-qPCR. The data are representative of three independent experiments. Data are expressed as mean ±SD. * Indicated p<0.05.
PDGFRα directly binds c-Kit and stimulates the expression of SCF
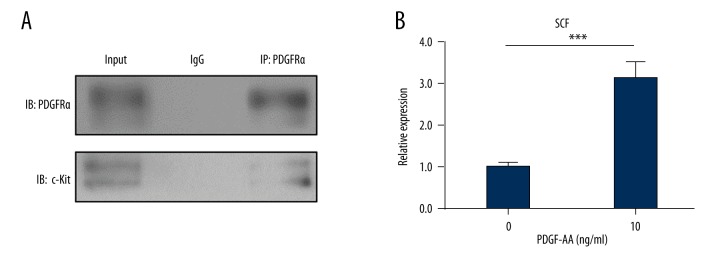
Some reports have shown that PDGFRα could bind and activate c-Kit kinase in gastrointestinal stromal tumors (GIST) [27–29]. To investigate whether PDGFRα binds c-Kit in cardiac fibroblasts, we performed the co-IP assay. As shown in Figure 5A, PDGFRα directly interacted with c-Kit in cardiac fibroblasts. Due to the important role of fibroblasts in production of SCF [30], we tested the mRNA level of SCF in the cardiac fibroblasts treated by PDGF-AA. As shown in Figure 5B, RT-qPCR results showed that PDGF-AA increased the mRNA level of SCF (p<0.001).
Figure 5.
PDGFRα directly binds c-Kit and stimulates the expression of SCF. (A) Cardiac fibroblasts were treated with PDGF-AA (10 ng/mL) for 24 hours. Lysates from the cardiac fibroblasts were immunoprecipitated with an anti-PDGFRα antibody or a nonspecific immunoglobulin G control (IgG), followed by immunoblotting with the anti-c-Kit antibody or anti-PDGFRα antibody. Protein input served as an immunoblotting control (input). (B) Cardiac fibroblasts were treated with PDGF-AA for 12 hours. The mRNA expression of SCF was tested by RT-qPCR. IP: immunoprecipitation; IB: immunoblotting. The data are representative of three independent experiments. Data are expressed as mean ±SD. *** Indicated p<0.001.
Discussion
Cardiac fibrosis is linked to many clinical cardiovascular diseases and pathological processes, and it causes heart failure, arrhythmia, sudden cardiac death, and other serious complications. Due to unclear mechanisms of cardiac fibrosis, there are currently no effective therapies to prevent its occurrence or halt its progression [2]. PDGF is known as an important activated agent for fibroblasts and an inducer of extracellular matrix protein synthesis (e.g., collagens, fibronectin, and proteoglycans) [31]. Overexpression of PDGFs in mice induces cardiac fibrosis [7]. PDGFRs are strongly expressed in fibroblasts [6,32]. After binding PDGFs, activated PDGFRs enhance fibroblast proliferation, migration, and differentiation [3]. However, the definite molecular mechanisms that PDGF/PDGFR signaling uses to stimulate the activation of cardiac fibroblast have not been uncovered.
This is the first study exploring the role of c-Kit in the activation of cardiac fibroblast induced by activated PDGFRα. The c-Kit signaling pathway is often activated and plays an important role during injury in various tissues [11]. C-Kit is expressed in the various cell types, including hematopoietic stem cells, mast cells, germ cells, vascular endothelial cells, and adult CPCs [11,15,33]. Some studies have shown that c-Kit was negative in adult cardiac fibroblasts but positive in neonatal cardiac fibroblasts [21,34]. Consistent with other report [21], our results showed that c-Kit was expressed in neonatal mouse cardiac fibroblasts. Hence, we used neonatal mouse cardiac fibroblasts in the in vitro experiment.
Numerous studies have shown that c-Kit positive CPCs played an important role in myocardial regeneration and repair [13,17,18,20]. In addition, c-Kit promoted growth and migration of CPCs through activating the PI3K/AKT and MEK/ERK pathways [13]. Our data showed that inhibition of c-Kit weakened the expressions of α-SMA and collagens in cardiac fibroblasts, caused by PDGF-AA. Activated PDGFRα could provoke downstream signaling pathways such as the MRK/ERK pathway, the PI3K/AKT pathway, and the STAT3 pathway [35]. So PI3K and ERK are the common downstream proteins of both PDGFRα and c-Kit in some cell types. These results indicate that activated c-Kit could accelerate the activation of cardiac fibroblasts.
PDGFRα and c-Kit are members of the PDGFR family and share a common topology consisting of an extracellular ligand-binding domain, a single spanning transmembrane domain, and an intracellular split kinase domain [9]. Some studies have shown that c-Kit could heterodimerize with PDGFR family members (e.g., FLT3, PDGFRα, and PDGFRβ) and activate them [27,28,36]. Similarly, PDGFRα binds and activates c-Kit in GIST cells [27]. In our study, we found that PDGFRα bound c-Kit, and c-Kit kinase was provoked in the activated cardiac fibroblasts. These results indicated that activated PDGFRα bound and activated c-Kit in cardiac fibroblasts.
Imatinib has been widely used and approved in the treatment of GIST and BCR-ABL positive leukemia (chronic myeloid leukemia), and has dramatically improved the clinical outcomes of those cancers [37,38]. Imatinib not only inhibits the kinases activation of c-Abl and PDGFRs but also restrains the activity of c-Kit [26]. Imatinib inhibited the BCR-ABL/c-Abl, c-Kit, and PDGFRα potently with an IC50 of 0.6, 0.1, and 0.1 μM, respectively [39]. This indicates that c-Kit and PDGFRα are more sensitive than BCR-ABL/c-Abl to imatinib. Indeed, the imatinib concentration required to inhibit the activity of c-Abl and achieve significant growth inhibitory effects in cells is at least 5 μM [40]. We used 0.5 μM and 1.0 μM imatinib to treat the activated cardiac fibroblasts and the activity of PDGFRα and c-Kit not c-Abl was inhibited.
The biologically active SCF is a homodimeric protein that is primarily produced by fibroblasts [30]. Our data demonstrated that the mRNA level of SCF was significantly increased in cardiac fibroblasts treated by PDGF-AA. SCF contributed to the migration of lung fibroblasts via an autocrine/paracrine mechanism [41]. These data indicated that the autocrine or paracrine mechanism of SCF may play an important role in the activation of c-Kit and the process of cardiac fibrosis. In the future, we will assess the role of autocrine or paracrine of SCF in activation of c-Kit caused by PDGF-AA/PDGFRα.
Conclusions
The results of this study have demonstrated that PDGF-AA/PDGFRα signaling activated cardiac fibroblasts by activating c-Kit. Our results illustrated the potential regulating effect of c-Kit in cardiac fibrosis, and provided new insight for activation of cardiac fibroblasts induced by PDGF-AA/PDGFRα signaling pathway. Meanwhile, this study indicated that c-Kit could be used as a potential therapeutic target for treatment fibrosis.
Acknowledgement
We thank the teachers of the Medical Science Experimentation Center, Zhongshan School of Medicine, Sun Yat-sen University for technical support.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by Natural Science Funds of China (81370215 and 81570039), and China Postdoctoral Science Foundation (2016M590838)
References
- 1.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117(3):568–75. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leask A. Getting to the heart of the matter: New insights into cardiac fibrosis. Circ Res. 2015;116(7):1269–76. doi: 10.1161/CIRCRESAHA.116.305381. [DOI] [PubMed] [Google Scholar]
- 3.Travers JG, Kamal FA, Robbins J, et al. Cardiac fibrosis: The fibroblast awakens. Circ Res. 2016;118(6):1021–40. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan Z, Guan J. Antifibrotic therapies to control cardiac fibrosis. Biomater Res. 2016;20(2):63–75. doi: 10.1186/s40824-016-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Cui Q, Su G, et al. MicroRNA-208b alleviates post-infarction myocardial fibrosis in a rat model by inhibiting GATA4. Med Sci Monit. 2016;29(22):1808–16. doi: 10.12659/MSM.896428. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Zhao TQ, Zhao WY, Chen YJ, et al. Platelet-derived growth factor-D promotes fibrogenesis of cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2013;304(12):H1719–26. doi: 10.1152/ajpheart.00130.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallini R, Lindblom P, Bondjers C, et al. PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice. Exp Cell Res. 2016;349(2):282–90. doi: 10.1016/j.yexcr.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Diez C, Nestler M, Friedrich U, et al. Down-regulation of Akt/PKB in senescent cardiac fibroblasts impairs PDGF-induced cell proliferation. Cardiovasc Res. 2001;49(4):731–40. doi: 10.1016/s0008-6363(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 9.Toffalini F, Demoulin JB. New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood. 2010;116(14):2429–37. doi: 10.1182/blood-2010-04-279752. [DOI] [PubMed] [Google Scholar]
- 10.Yuzawa S, Opatowsky Y, Zhang Z, et al. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell. 2007;130(2):323–34. doi: 10.1016/j.cell.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, Wu YL, Chen BJ, et al. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci. 2013;9(5):435–43. doi: 10.7150/ijbs.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai Y, Huang Y, Tang H, et al. Role of stem cell growth factor/c-Kit in the pathogenesis of irritable bowel syndrome. Exp Ther Med. 2017;13(4):1187–93. doi: 10.3892/etm.2017.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vajravelu BN, Hong KU, Al-Maqtari T, et al. C-Kit promotes growth and migration of human cardiac progenitor cells via the PI3K-AKT and MEK-ERK pathways. PLoS One. 2015;10(10):e0140798. doi: 10.1371/journal.pone.0140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui J, Wakabayashi T, Asada M, et al. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279(18):18600–7. doi: 10.1074/jbc.M311643200. [DOI] [PubMed] [Google Scholar]
- 15.Thoren LA, Liuba K, Bryder D, et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180(4):2045–53. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 16.Ledford BT, Simmons J, Chen M, et al. Keratose hydrogels promote vascular smooth muscle differentiation from C-kit-positive human cardiac stem cells. Stem Cells Dev. 2017;26(12):888–900. doi: 10.1089/scd.2016.0351. [DOI] [PubMed] [Google Scholar]
- 17.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121(18):1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 19.Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120(12):1075–83. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nigro P, Perrucci GL, Gowran A, et al. c-kit(+) cells: The tell-tale heart of cardiac regeneration? Cell Mol Life Sci. 2015;72(9):1725–40. doi: 10.1007/s00018-014-1832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y, Guo K, Li Q, et al. Multiple directional differentiation difference of neonatal rat fibroblasts from six organs. Cell Physiol Biochem. 2016;39(1):157–71. doi: 10.1159/000445613. [DOI] [PubMed] [Google Scholar]
- 22.Wang LX, Yang X, Yue Y, et al. Imatinib attenuates cardiac fibrosis by inhibiting platelet-derived growth factor receptors activation in isoproterenol induced model. PLoS One. 2017;12(5):e0178619. doi: 10.1371/journal.pone.0178619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan GP, Wang W, Zhao H, et al. Pharmacological inhibition of focal adhesion kinase attenuates cardiac fibrosis in mice cardiac fibroblast and post-myocardial-infarction models. Cell Physiol Biochem. 2015;37(2):515–26. doi: 10.1159/000430373. [DOI] [PubMed] [Google Scholar]
- 24.Luo L, Zhang C, Zhao J, et al. Effects of rapamycin on reduction of peridural fibrosis: An experimental study. Med Sci Monit. 2015;13(21):482–88. doi: 10.12659/MSM.893165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimminger F, Schermuly RT. PDGF receptor and its antagonists: Role in treatment of PAH. Adv Exp Med Biol. 2010;661:435–46. doi: 10.1007/978-1-60761-500-2_28. [DOI] [PubMed] [Google Scholar]
- 26.Grimminger F, Schermuly RT, Ghofrani HA. Targeting non-malignant disorders with tyrosine kinase inhibitors. Nat Rev Drug Discov. 2010;9(12):956–70. doi: 10.1038/nrd3297. [DOI] [PubMed] [Google Scholar]
- 27.Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology. 2003;125(3):660–67. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhu MJ, Ou WB, Fletcher CD, et al. KIT oncoprotein interactions in gastrointestinal stromal tumors: Therapeutic relevance. Oncogene. 2007;26(44):6386–95. doi: 10.1038/sj.onc.1210464. [DOI] [PubMed] [Google Scholar]
- 29.Negri T, Bozzi F, Conca E, et al. Oncogenic and ligand-dependent activation of KIT/PDGFRA in surgical samples of imatinib-treated gastrointestinal stromal tumours (GISTs) J Pathol. 2009;217(1):103–12. doi: 10.1002/path.2450. [DOI] [PubMed] [Google Scholar]
- 30.Hesse M, Fleischmann BK, Kotlikoff MI. The role of C-kit expressing cells in heart repair at the neonatal and adult stage. Stem Cells. 2014;32(7):1701–12. doi: 10.1002/stem.1696. [DOI] [PubMed] [Google Scholar]
- 31.Kazlauskas A. PDGFs and their receptors. Gene. 2017;614:1–7. doi: 10.1016/j.gene.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore-Morris T, Cattaneo P, Puceat M, Evans SM. Origins of cardiac fibroblasts. J Mol Cell Cardiol. 2016;91:1–5. doi: 10.1016/j.yjmcc.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keith MCL, Bolli R. “String theory” of c-kit(pos) cardiac cells a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results”. Circ Res. 2015;116(7):1216–30. doi: 10.1161/CIRCRESAHA.116.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bei Y, Zhou Q, Fu S, et al. Cardiac telocytes and fibroblasts in primary culture: Different morphologies and immunophenotypes. PLoS One. 2015;10(2):e0115991. doi: 10.1371/journal.pone.0115991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ekpe-Adewuyi E, Lopez-Campistrous A, Tang X, et al. Platelet derived growth factor receptor alpha mediates nodal metastases in papillary thyroid cancer by driving the epithelial-mesenchymal transition. Oncotarget. 2016;7(50):83684–700. doi: 10.18632/oncotarget.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otto KG, Jin LQ, Spencer DM, Blau CA. Cell proliferation through forced engagement of c-Kit and Flt-3. Blood. 2001;97(11):3662–64. doi: 10.1182/blood.v97.11.3662. [DOI] [PubMed] [Google Scholar]
- 37.Stagno F, Stella S, Spitaleri A, et al. Imatinib mesylate in chronic myeloid leukemia: frontline treatment and long-term outcomes. Expert Rev Anticancer Ther. 2016;16(3):273–78. doi: 10.1586/14737140.2016.1151356. [DOI] [PubMed] [Google Scholar]
- 38.Milhem M, Deutsch JM. Imatinib dosing in gastrointestinal stromal tumors (GISTs): When, how much, and how long? Curr Clin Pharmacol. 2015;10(4):311–20. doi: 10.2174/1574884710666151020100518. [DOI] [PubMed] [Google Scholar]
- 39.Nimmanapalli R, Porosnicu M, Nguyen D, et al. Cotreatment with STI-571 enhances tumor necrosis factor alpha-related apoptosis-inducing ligand (TRAIL or apo-2L)-induced apoptosis of Bcr-Abl-positive human acute leukemia cells. Clin Cancer Res. 2001;7(2):350–57. [PubMed] [Google Scholar]
- 40.Lupino E, Ramondetti C, Buccinnà B, Piccinini M. Exposure of neuroblastoma cell lines to imatinib results in the upregulation of the CDK inhibitor p27(KIP1) as a consequence of c-Abl inhibition. Biochem Pharmacol. 2014;92(2):235–50. doi: 10.1016/j.bcp.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed Abdi B, Lopez H, Karrar S, et al. Use of patterned collagen coated slides to study normal and scleroderma lung fibroblast migration. Sci Rep. 2017;7(1):2628. doi: 10.1038/s41598-017-02621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]