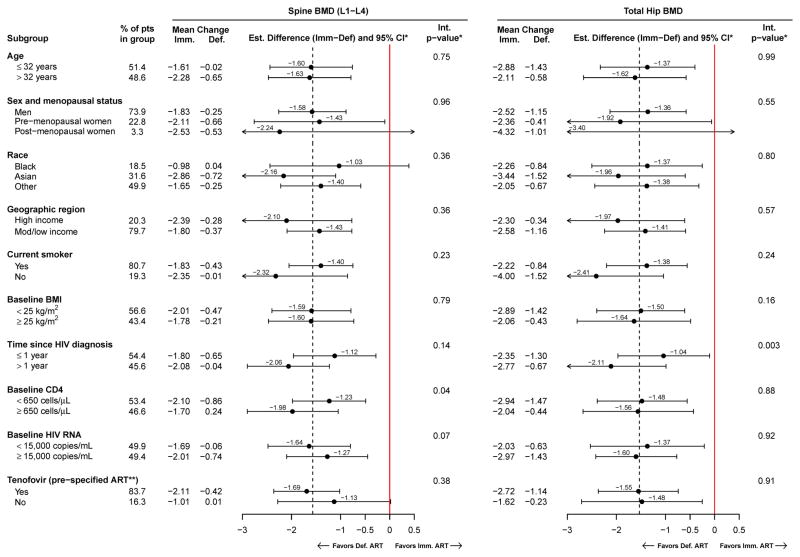
Figure 3.
Subgroup analyses: Mean percent change from baseline, and treatment differences (immediate minus deferred ART groups) in spine (L1–L4) and total hip BMD are estimated within subgroups, with 95% confidence intervals. P-values are for tests of heterogeneity of the treatment difference across subgroups.
* Estimated in a longitudinal mixed model, adjusted for visit and baseline BMD. The interaction p-value for heterogeneity across subgroups was calculated using continuous variables for age, BMI, time since HIV diagnosis, CD4 count, and log10 HIV RNA levels.
** In the immediate ART group, a tenofovir-containing ART regimen was selected prior to randomization (“pre-specified”) for 162 participants. Of those who were pre-specified tenofovir and had the corresponding follow-up scans, 155 (96.3%).138 (97.2%), and 45 (97.8%) used tenofovir at years 1, 2, and 3, respectively.