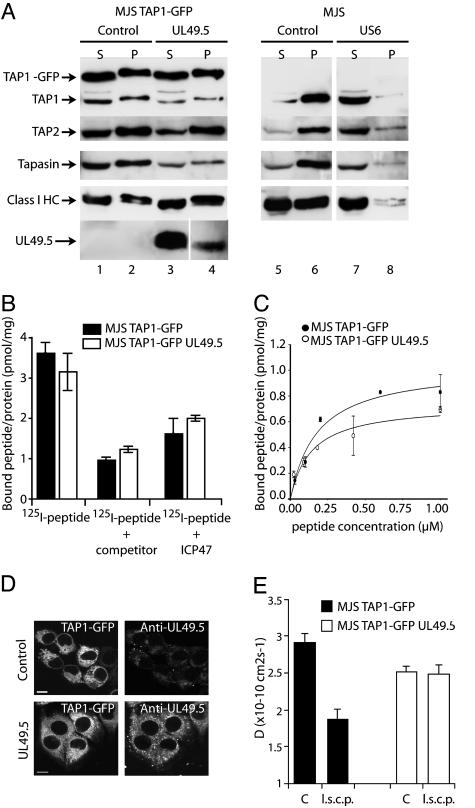
Fig. 6.
UL49.5 does not block ATP or peptide binding by TAP but arrests the transporter in a translocation-incompetent state. (A) Postnuclear supernatants of digitonin-solubilized cells were incubated with ATP-agarose. Equal cell equivalents of the ATP-agarose bound pellet (P) and unbound supernatant (S) fractions were separated by SDS/PAGE and immunoblotted with Abs against the proteins indicated. (B and C) Peptides can bind to TAP in the presence of UL49.5. (B) Microsomal membranes were incubated with 1 μM 125I-labeled peptide RRYQKSTEL in the absence or presence of 400 μM unlabeled RRYQKSTEL (competitor) or in the presence of 200 μM ICP47. Each data point represents the mean of three measurements. (C) Microsomal membranes were incubated with increasing concentrations of the 125I-labeled peptide RRYQSTEL. Unspecific binding was determined in the presence of 200-fold excess of ICP47. The amount of specifically bound peptide per amount of microsomal protein is plotted against the peptide concentration and fitted with a 1:1 Langmuir equation (44). (D) TAP1–GFP (Left) and UL49.5 distribution (Right) in MJS TAP1–GFP cells. Cells were fixed and stained with anti-UL49.5 antibodies. (Scale bar: 5 μm.) (E) Analysis of TAP activity in vivo as measured by lateral mobility of the TAP complex (D, diffusion coefficient). Cells were microinjected with long side-chain peptides (l.s.c.p.) as indicated. C, control.