Fig. 2.
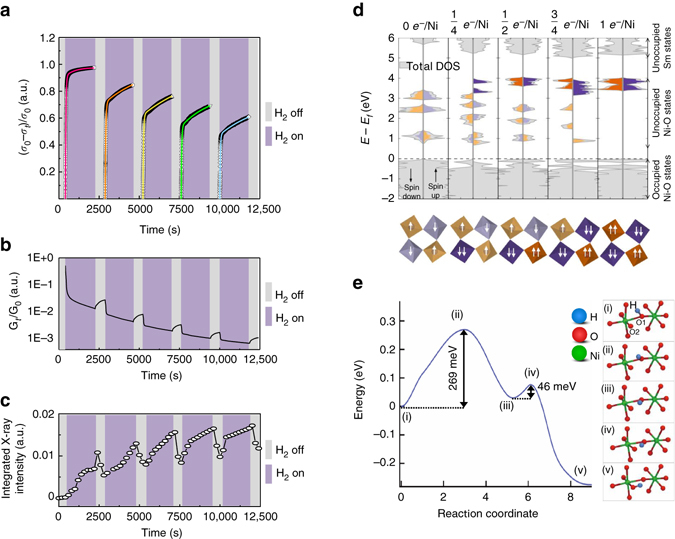
Mechanism of habituation in a perovskite nickelate. a In situ visualization of habituation phenomenon, i.e., exponential decrease of conductivity change upon environmental exposure (the dots represent the experimental data and the solid lines are fits.). σ 0 and σ t are initial and dynamical conductivity, respectively. b The conductance changes in response to different environments (decrease in H2 and increase in air) showing inherent plasticity similar to what is observed in biological synapses. G 0 and G t represent initial and dynamical conductance, respectively. c Structural lattice breathing monitored by in situ synchrotron X-ray diffraction. The integrated intensities of x-ray diffraction peak at q z = 2.98 Å−1 related to H-SmNiO3 (H-SNO) are shown (see Supplementary Fig. 4). d First-principles calculation of electron-doped SNO. The upper figure shows density of states (DOS), in gray, at different doping levels from 0-1 added e − per Ni site. The unoccupied projected DOS (PDOS) on each nickel site is shown in orange and purple. The difference in the total DOS and the PDOS is due to the strong hybridization of the Ni and O states resulting from the covalent nature of the NiO6 octahedra. The lower figure shows the occupied Ni e g levels for the corresponding doping levels. Same color legend is used and the darker colors indicate Ni with two occupied e g states. e Atomic-scale pathway, and the associated energy barriers for proton migration between two neighboring O atoms labeled as O1 and O2 in (i) within a NiO6 octahedron in a monoclinic SNO crystal. The potential energy along the most preferred diffusion pathway (as obtained from nudged-elastic band density functional theory (DFT) calculation) is shown on the left, while selected configurations along this pathway labeled (i)–(v) are depicted on the right
