Abstract
We aim to find the risk factors that influence the formation of bladder calculi in patients with benign prostate hyperplasia (BPH) and to reduce the surgical intervention related to bladder calculi.
Between January 2015 and October 2016, 332 patients with BPH underwent surgical therapy were retrospectively evaluated. Patients with BPH were categorized into 2 groups: 94 patients with bladder calculi in group 1 and 238 patients without bladder calculi in group 2. Medical history, age, body mass index (BMI), total prostate specific antigen, total prostate volume (TPV), International Prostate Symptom Score (IPSS), intravesical prostatic protrusion (IPP), urodynamic parameters, and urine culture were compared between groups.
There was no significant difference in the age, BMI, peak flow rate, and total IPSS between groups. TPV, total prostate specific antigen, and duration of BPH were significantly lower in group 1 than those in group 2. In addition, IPP was significantly higher in group 1 than group 2 (P < .001). Besides, after exclusion of patients with urinary retention and indwelling catheter, group 1 associated with a significantly higher preoperative positive rate of urine culture than that of group 2 (P = .046). Multivariate analysis indicated that IPP was a significant independent risk factor for the presence of bladder calculi.
The incidence of bladder calculi in patients with BPH was proved to be closely associated with preoperative positive urine culture and longer IPP in our study. Furthermore, the IPP was presented to be an independent risk factor for the formation of bladder calculi. And early antibacterial therapy of urinary tract infection (UTI) may help to prevent the presence of bladder calculi in patients with BPH.
Keywords: benign prostatic hyperplasia, bladder calculi, intravesical prostatic protrusion, urinary retention, urine culture
1. Introduction
Bladder calculi is one of the common complications of benign prostatic hyperplasia (BPH) and the incidence is about 10%.[1] The presence of bladder calculi in patients with BPH is an absolute indication for surgery.[2,3] The bladder calculi not only increases patients’ sufferings and medical expenses, but also may be associated with the bladder cancer.[4] It is generally accepted that urinary retention secondary to bladder outlet obstruction (BOO) is an important cause for the formation of bladder calculi in patients with BPH. But, the incidence of bladder calculi related to urinary retention only accounts for 3% to 8% of patients with BOO due to BPH.[5–7] Some studies reported that the formation of calculi is complex, and the urinary retention alone may not lead to the urolithiasis.[8–10] The etiology of bladder calculi in men with BPH is still not clear. The aim of this study is to assess the risk factors that influence the formation of bladder calculi in patients with BPH and to reduce the rate of surgical intervention related to the bladder calculi.
2. Materials and methods
2.1. Study population
We designed a retrospective study to compare patients underwent selected surgical intervention for BPH with and without bladder calculi. Between January 2015 and October 2016, a total of 332 patients with BPH underwent surgical therapy in our hospital were included. They were categorized into 2 groups: group 1 had bladder calculi and group 2 did not. All participants were given written informed consent, and all procedures performed in study in accordance with the ethical standards of the First Affiliated Hospital, Zhejiang University, School of Medicine and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
2.2. Inclusion criteria
Patients were enrolled if they underwent transurethral prostate resection for BPH and had a pathologic verification. Exclusion criteria: patients were excluded if they had been confirmed prostate malignancy, neurogenic bladder, bladder foreign body, hydronephrosis, previous history of urolithiasis, or urethral stricture.
2.3. Clinical data
Preoperative date collection included age, body mass index (BMI), medical history of BPH, serum prostate specific antigen (PSA), the International Prostate Symptom Score (IPSS), urodynamic examination, urine culture, and transabdominal ultrasonography. The total prostate volume (TPV), intravesical prostatic protrusion (IPP), and the maximum diameter and number of bladder calculi were measured on the supine patients by the same physician using the transabdominal ultrasonography in the condition of proper filling (150–200 mL) of the bladder. The diameter, anteroposterior diameter, and superoinferior diameter were measured firstly, then, the vertical distance from the highest point of the protruding prostate to the bladder neck was measured as the IPP length by the median longitudinal sagittal plane.[11] Under the recommendations of the International Continence Society, urodynamic examination was performed to record the peak flow rate and postvoid residual (PVR) urine volume.[12] All the patients underwent transuretheral resection of prostate with or without endoscopic lithotripsy.
2.4. Statistical analysis
Statistical analysis was done using SPSS 16.0. Values are presented as the mean ± standard deviation. Continuous variables were compared by the Student t test or the Mann–Whitney U test. Categorical variables were analyzed by the Pearson chi-square tests. Logistic regression analyses were used to determine whether age, BMI, PSA, IPSS, TPV, IPP, or the peak flow rate was an independent risk factor of the presence of bladder calculi in patients with BPH. A P value < .05 was considered to be statistically significant.
3. Results
Of the 332 patients with BPH enrolled in the study, patients with bladder calculi were classified as group 1 (n = 94) and patients without bladder calculi were classified as group 2 (n = 238). The mean age of patients was 68.5 ± 7.6 years (range 53–88 years) versus 69.1 ± 7.8 years (range 50–89 years) in group 1 and 2 (P = .51), respectively (Table 1). According to the outcomes of transabdominal ultrasonography, the maximum diameter of the bladder calculi was 2.1 ± 1 cm, and 66% of the patients had at least 2 stones in group 1. No significant differences were found in BMI, total IPSS, peak flow rate, and PVR between 2 groups.
Table 1.
Comparison of baseline characteristics between group 1 and group 2.
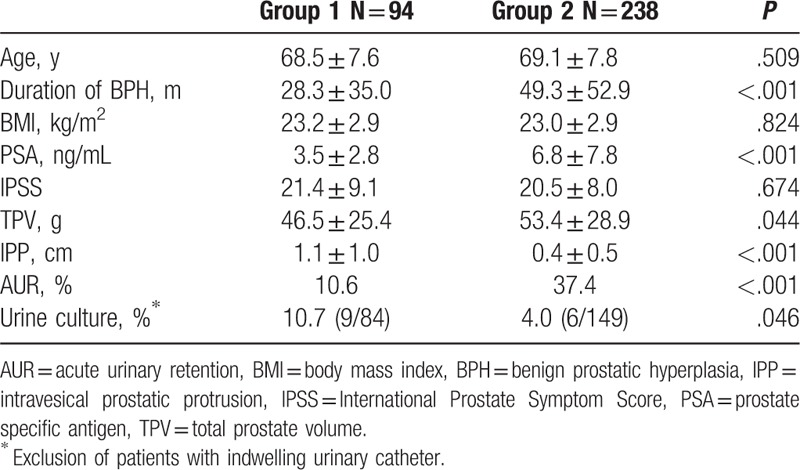
However, group 1 presented a significantly shorter duration of diagnosis of BPH until the time of transuretheral resection of prostate than that of group 2 (28.3 ± 35 vs 49.3 ± 52.9 months, P < .001). Incidence of acute urinary retention (AUR) in group 1 (10.6%) was also lower than that of group 2 (37.4%). After exclusion of patients with indwelling catheter, a significant difference in the positive rate of urine culture was noted between group 1 and 2 (10.7% vs 4%, P = .046). The most common bacteria detected in group 1 were Enterococcus faecalis and Staphylococcus epidermidis, while E faecalis, Pseudomonas aeruginosa, and Escherichia coli were in group 2.
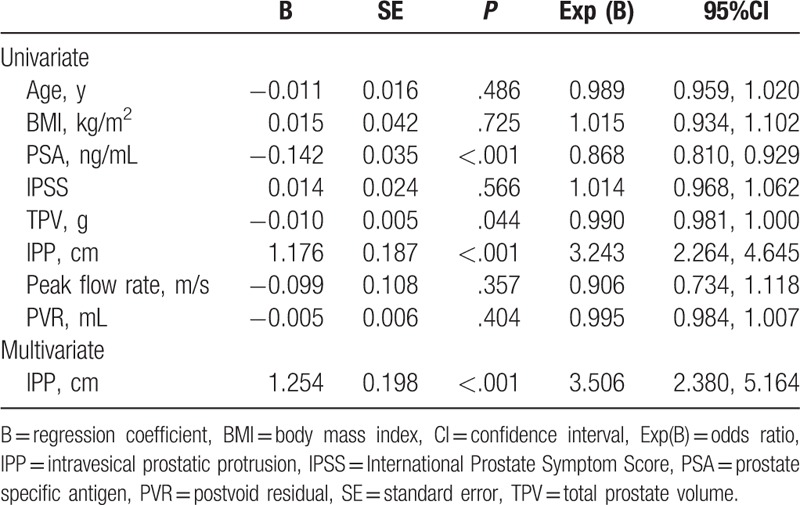
The TPV and total prostate specific antigen were significantly lower in group 1 than those of group 2. Meanwhile, the IPP was significantly longer in group 1 than that of group 2 (P < .001). Univariate and multivariate analysis showed that IPP (HR = 3.506, P < .001) was an independent risk factor of the presence of bladder calculi in patients with BPH (Table 2).
Table 2.
Univariate and multivariate analysis of data between group 1 and group 2.
4. Discussion
Bladder calculi is one of the most common urolithiasis in men over 50 years old, and it could be classified as primary, secondary, and migratory stones. And some authors have proposed that the incidence of bladder calculi is mainly associated with BOO, bladder augmentation, bladder diverticula, urinary tract infection (UTI), neurogenic bladder, or the bladder foreign body.[13] Generally, BOO associated with prostatic hyperplasia may induce UTI, which could act as initial factor of lithogenesis, especially when the PVR urine volume increased because of poor bladder emptying.[14] These would lead to the deposition of small crystalline in urine, which could serve as a core for the formation of bladder calculi. Apart from obstruction and infection, metabolic and nutritional disorders were also reported to be related to the formation of bladder calculi.[14,15]
IPP is an anatomical structure of the prostate caused by the enlargement of the medial and/or lateral lobes of prostate, referring to the vertical distance from the top of the protruding prostate to the bladder base. Chia et al[16] reported that IPP could act as a clinical indicator to determine the degree of BOO. Lim et al[17] reported that long IPP would cause BOO by exerting “ball valve” effect on the process of urination, and the funnel effect of the bladder neck would be destroyed. And furthermore, the correlation between IPP and the degree of BOO was proposed to be more reliable than that between other factors (such as IPSS, TPV, peak flow rate, and total prostate specific antigen) and BOO.[18]
Our study revealed that IPP was significantly longer in group 1 than group 2, which is consistent with the finding of previous study.[18] Moreover, the results of univariate and multivariate analysis in our study showed that IPP was an independent risk factor of the presence of bladder calculi in patients with BPH, which further indicated that IPP was closely associated with the formation of bladder calculi.
Interestingly, we found the incidence of AUR in group 1 was lower than that of group 2, which could mean that the mechanism of stone formation is multifactorial. AUR with indwelling catheter could increase the positive rate of urine culture. To avoid the bias, patients with indwelling catheters were excluded when we evaluated the positive rate of urine culture. We found the preoperative positive rate of urine culture was higher in group 1 than that of group 2 (P = .046). BPH patients with bladder calculi may be more likely to have a positive urine culture. We assumed that UTI caused by bacteria may alter urinary PH and citrate concentration, which could contribute to the formation of crystal. Moreover, the change of urinary micro-environment associated with bacteria may be the underlying mechanism of bladder calculi. However, the data in our study are limited and the mechanisms of how bacteria leading to bladder stone are still not obscure. And more further studies are still needed to be investigated.
Our study also revealed that the TPV, PSA, and disease duration were significantly lower in group 1 than those of group 2. As bladder calculi was an absolute indication for the surgical treatment of BPH, patients in group 1 might undergo surgical therapy earlier than those of group 2, which might contribute to the lower TPV and disease duration in group 1. The level of PSA could be influenced by many factors, such as age, TPV, UTI, and indwelling catheter. A higher TPV and incidence of indwelling catheter in group 2 might contribute to a higher level of PSA value in this group.
As many studies reported that metabolic abnormalities were associated with lithogenesis,[10,19] we hypothesized the change of urinary micro-environment is also a risk factor for the pathogenesis of bladder calculi. However, it needs to be confirmed by trials in the future.
According to our study, the incidence of bladder calculi in patients with BPH was tended to be closely associated with preoperative positive urine culture and longer IPP in our study. Furthermore, the IPP was presented to be an independent risk factor for the formation of bladder calculi. Above all, we proposed that BPH patients with great IPP may benefit from early surgical intervention. Besides, the prevention of UTI is also important for the prevention of bladder calculi.
However, the main aim of our study is to reduce the surgical intervention related to bladder calculi in patients with BPH but not to increase the rate of early surgical intervention. As a main risk factor for the presence of bladder calculi in patients with BPH, the presence and lengthening of IPP is difficult to be prevented. Recently, a novel study reported that the degree of IPP could not be reduced by combining therapy using the with α-blocker and 5α-reductase.[20] And IPP could also contribute to the failure of medication and tend to increase the risk of progression in patients with BPH.[20,21] But the prevention and treatment of UTI is easy to be achieved. By performing the urine routine and urine culture in BPH patients complaining about LUTS or other associated symptoms, we are able to confirm the diagnosis of UTI early. And these will help us to select the proper antimicrobials and complete antibacterial therapy effectively, which may help to prevent the presence of bladder calculi in patients with BPH.
There are some limitations in our study. First, this study was retrospective, which might introduce recall bias. And the sample size included into our study is small. Second, the preoperative urine culture was only conducted once, so the results of which may have contingency. Third, some included patients did not have urine culture. Besides, quite a few patients lacked urodynamic data before surgery. Prospective randomized controlled trials are needed to confirm our conclusions.
5. Conclusions
In conclusion, the incidence of bladder calculi in patients with BPH was proved to be closely associated with preoperative positive urine culture and longer IPP in our study. Furthermore, the IPP was presented to be an independent risk factor for the formation of bladder calculi. And early antibacterial therapy of UTI may help to prevent the presence of bladder calculi in patients with BPH.
Footnotes
Abbreviations: AUR = acute urinary retention, BMI = body mass index, BOO = bladder outlet obstruction, BPH = benign prostate hyperplasia, IPP = intravesical prostatic protrusion, IPSS = International Prostate Symptom Score, PVR = postvoid residual, TPV = total prostate volume, UTI = urinary tract infection.
W-H and J-JC contributed equally to this work.
Funding/support: This study was funded by the National Natural Science Foundation of China (grant number 81370799) and Chinese Medicine Research Program of Zhejiang Province (grant number N20100606).
The authors have no conflicts of interest.
References
- [1].Papatsoris AG, Varkarakis I, Dellis A, et al. Bladder lithiasis: from open surgery to lithotripsy. Urol Res 2006;34:163–7. [DOI] [PubMed] [Google Scholar]
- [2].Gratzke C, Bachmann A, Descazeaud A, et al. EAU Guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2015;67:1099–109. [DOI] [PubMed] [Google Scholar]
- [3].McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011;185:1793–803. [DOI] [PubMed] [Google Scholar]
- [4].Philippou P, Moraitis K, Masood J, et al. The management of bladder lithiasis in the modern era of endourology. Urology 2012;79:980–6. [DOI] [PubMed] [Google Scholar]
- [5].Elzayat EA, Elhilali MM. Holmium laser enucleation of the prostate (HoLEP): the endourologic alternative to open prostatectomy. Eur Urol 2006;49:87–91. [DOI] [PubMed] [Google Scholar]
- [6].Mebust WK, Holtgrewe HL, Cockett AT, et al. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. 1989. J Urol 2002;167:999–1003. [PubMed] [Google Scholar]
- [7].Krambeck AE, Handa SE, Lingeman JE. Experience with more than 1,000 holmium laser prostate enucleations for benign prostatic hyperplasia. J Urol 2010;183:1105–9. [DOI] [PubMed] [Google Scholar]
- [8].Matlaga BR, Miller NL, Terry C, et al. The pathogenesis of calyceal diverticular calculi. Urol Res 2007;35:35–40. [DOI] [PubMed] [Google Scholar]
- [9].Husmann DA, Milliner DS, Segura JW. Ureteropelvic junction obstruction with a simultaneous renal calculus: long-term follow-up. J Urol 1995;153:1399–402. [PubMed] [Google Scholar]
- [10].Raj GV, Auge BK, Assimos D, et al. Metabolic abnormalities associated with renal calculi in patients with horseshoe kidneys. J Endourol 2004;18:157–61. [DOI] [PubMed] [Google Scholar]
- [11].Nose H, Foo KT, Lim KB, et al. Accuracy of two noninvasive methods of diagnosing bladder outlet obstruction using ultrasonography: intravesical prostatic protrusion and velocity-flow video urodynamics. Urology 2005;65:493–7. [DOI] [PubMed] [Google Scholar]
- [12].Griffiths D, Hofner K, van Mastrigt R, et al. Standardization of terminology of lower urinary tract function: pressure-flow studies of voiding, urethral resistance, and urethral obstruction. International Continence Society Subcommittee on Standardization of Terminology of Pressure-Flow Studies. Neurourol Urodyn 1997;16:1–8. [DOI] [PubMed] [Google Scholar]
- [13].Yoshida O, Okada Y. Epidemiology of urolithiasis in Japan: a chronological and geographical study. Urol Int 1990;45:104–11. [DOI] [PubMed] [Google Scholar]
- [14].Childs MA, Mynderse LA, Rangel LJ, et al. Pathogenesis of bladder calculi in the presence of urinary stasis. J Urol 2013;189:1347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park S, Pearle MS. Pathophysiology and management of calcium stones. Urol Clin North Am 2007;34:323–34. [DOI] [PubMed] [Google Scholar]
- [16].Chia SJ, Heng CT, Chan SP, et al. Correlation of intravesical prostatic protrusion with bladder outlet obstruction. BJU Int 2003;91:371–4. [DOI] [PubMed] [Google Scholar]
- [17].Lim KB, Ho H, Foo KT, et al. Comparison of intravesical prostatic protrusion, prostate volume and serum prostatic-specific antigen in the evaluation of bladder outlet obstruction. Int J Urol 2006;13:1509–13. [DOI] [PubMed] [Google Scholar]
- [18].Kim JW, Oh MM, Park HS, et al. Intravesical prostatic protrusion is a risk factor for bladder stone in patients with benign prostatic hyperplasia. Urology 2014;84:1026–9. [DOI] [PubMed] [Google Scholar]
- [19].Schwartz BF, Stoller ML. The vesical calculus. Urol Clin North Am 2000;27:333–46. [DOI] [PubMed] [Google Scholar]
- [20].Liu Q, Zhu Y, Liu J, et al. Ultrasound image features of intravesical prostatic protrusion indicated failure of medication therapy of finasteride and doxazosin in patients with benign prostatic hyperplasia (LUTS/BPH). Int Urol Nephrol 2017;49:399–404. [DOI] [PubMed] [Google Scholar]
- [21].Matsukawa Y, Ishida S, Majima T, et al. Intravesical prostatic protrusion can predict therapeutic response to silodosin in male patients with lower urinary tract symptoms. Int J Urol 2017;24:454–9. [DOI] [PubMed] [Google Scholar]


