Figure 1. Glutamine metabolism in sprouting endothelial cells.
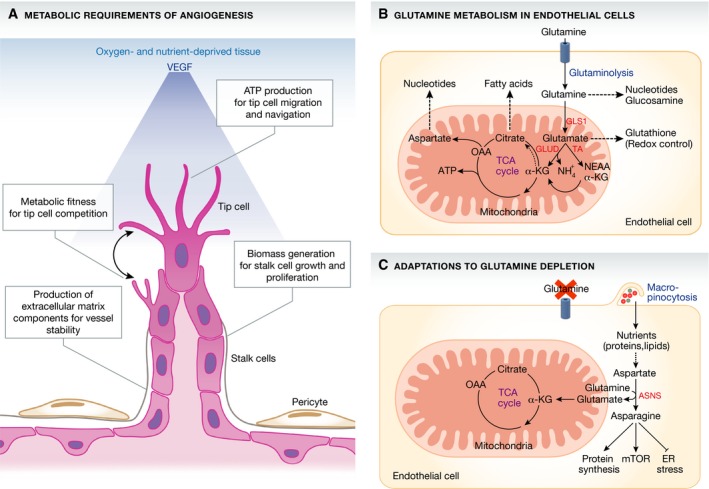
(A) ECs are confronted with several metabolic challenges when they invade avascular tissues. A gradient (blue) of VEGF, secreted by the nutrient‐ and oxygen‐deprived tissue, activates ECs to sprout, migrate and proliferate. Sprouting ECs need to increase their biosynthetic activity to produce sufficient energy (ATP) and biomass (nucleotides, protein and lipids) required for vascular expansion. (B) Huang et al (2017) and Kim et al (2017) show that ECs consume large amounts of glutamine to produce nucleotides, amino acids and lipids required for cell growth and duplication. Endothelial GLS1 activity was found to be essential to replenish the TCA cycle, to produce biomass and to maintain redox balance. Dashed arrow within the TCA cycle indicates reductive carboxylation, which denotes the carboxylation of α‐KG to citrate. (C) Under conditions of glutamine limitation, ECs can in part compensate for the lack of glutamine catabolism by using alternative nutrient acquisition pathways. When glutamine levels drop, ECs use macropinocytosis to take up extracellular nutrients such as asparagine. ECs use asparagine for protein synthesis, reactivation of mTOR signalling and the suppression of endoplasmic reticulum (ER) stress. EC, endothelial cell; VEGF, vascular endothelial growth factor; ATP, adenosine triphosphate; GLS1, glutaminase 1; GLUD, glutamate dehydrogenase; TA, transaminases; NEAA, non‐essential amino acids; α‐KG, α‐ketoglutarate; NH4 +, ammonium; OAA, oxaloacetate; ASNS, asparagine synthethase; mTOR, mechanistic target of rapamycin; ER, endoplasmic reticulum.
