Figure 8. Model for HORMA domain remodeling by MAD2.
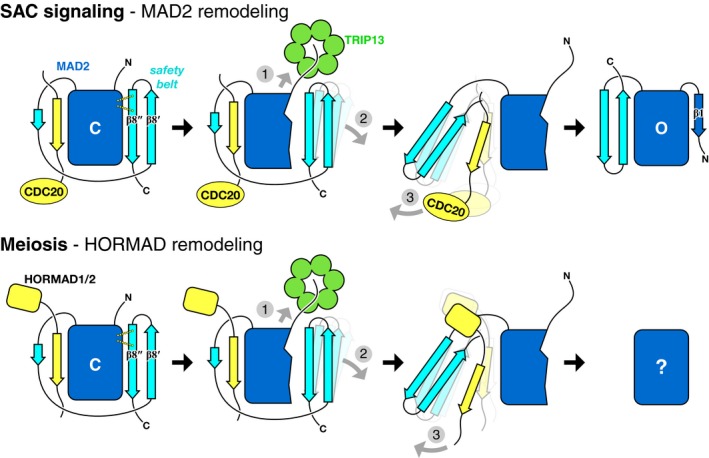
Proposed mechanism of TRIP13 in the spindle assembly checkpoint (top) and meiosis (bottom). In both cases, TRIP13 (green) recognizes HORMA domain proteins (MAD2 or meiotic HORMADs; blue) in the closed conformation, bound to closure motifs of binding partners (yellow; CDC20 to MAD2, HORMAD C‐termini for HORMADs). Limited unfolding of the HORMA domain N‐terminus disrupts a hydrogen‐bond network stabilizing the C‐terminal safety belt (1), mediating its dissociation from the HORMA domain core (2), followed by release of the bound closure motif (3). Following release of the MAD2 N‐terminus by TRIP13, this region forms strand β1 to stabilize the open conformation. The conformation of unbound meiotic HORMADs is currently unknown.
