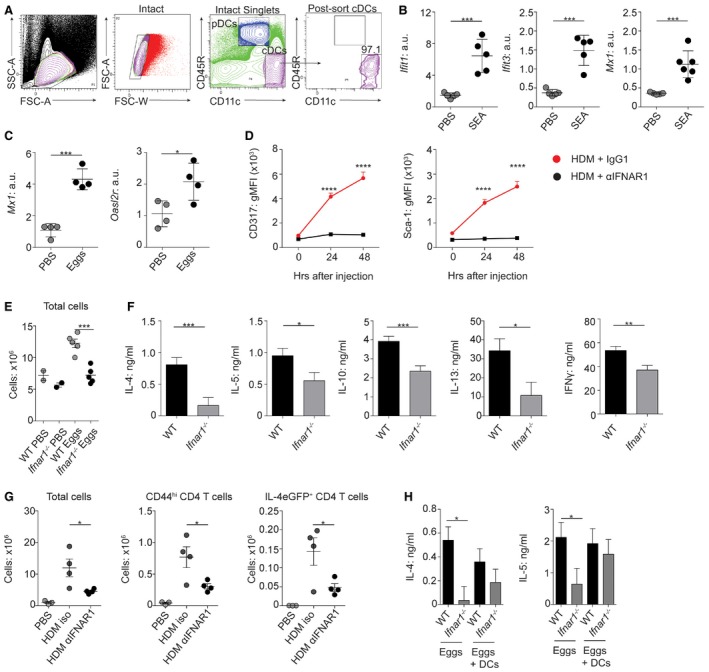
-
A
Splenic DCs were FACS‐isolated following 12‐h exposure to 50 μg SEA administered i.v. Cells were gated as intact singlets and cDCs identified as CD45R− CD11c+ cells sorted to > 95% purity.
-
B
mRNA expression of ISGs by splenic sorted cDCs was assessed by qPCR (normalized against Gapdh, a.u.) following in vivo exposure to SEA.
-
C
mRNA expression of ISGs in whole lung following pulmonary challenge with Schistosoma mansoni eggs (normalized against beta‐actin, a.u.).
-
D
Mice were challenged i.d. with 100 μg HDM in conjunction with 200 μg of the IFNAR1‐blocking Ab MAR1‐5A3 or an isotype control. Twenty‐four or 48 h later, auricular LNs were harvested and CD317 and Sca‐1 expression on cDCs analyzed.
-
E, F
A total of 2,500 S. mansoni eggs were injected s.c. per foot into WT or Ifnar1
−/− mice, dLNs were harvested 7 days later, and cell counts were performed (E). (F) Cells were cultured with anti‐CD3 for 72 h and then cytokines measured by ELISA (medium background subtracted).
-
G
IL‐4eGFP mice were challenged i.d. with 100 μg HDM in conjunction with 200 μg of the IFNAR1‐blocking Ab MAR1‐5A3 or an isotype control. Mice were given a second dose of Ab i.p. 48 h later. On day 7, dLNs were harvested and total cell numbers, CD44hi CD4+ T cell numbers, and IL‐4eGFP+ T cell numbers assessed.
-
H
A total of 2.5 × 103
S. mansoni eggs were injected s.c. with (+DC) or without 1 × 106 WT FLDCs into WT or Ifnar1
−/− recipients. On day 7, dLNs were harvested, cells were stimulated with anti‐CD3 for 72 h, and cytokines were measured by ELISA (medium background subtracted).
Data information: Results are mean ± SEM (B–E, G) (one‐way ANOVA) or least squares mean ± SEM (F, H) (analyzed using a three‐way full‐factorial fit model, with contrast analysis used to test differences between experimental groups). *
P < 0.05, **
P < 0.01, ***
P < 0.001, ****
P < 0.0001. Data from one of three or more experiments (A–E, G) (
n = 2–5 animals per group, five replicate wells), or data from three (H) or six (F) experiments pooled. a.u., arbitrary units.