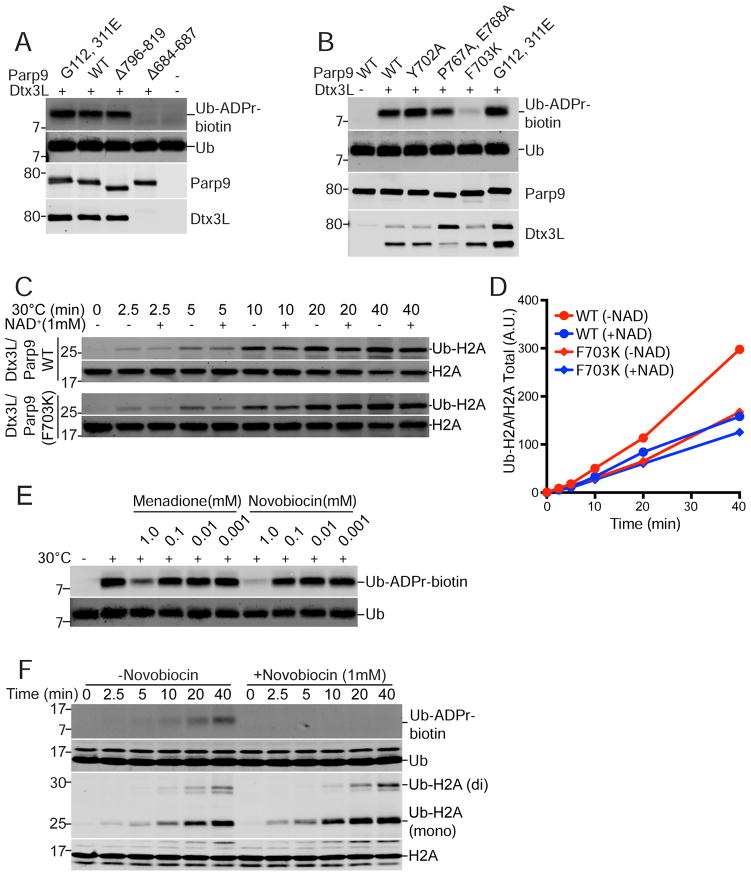
Figure 3. Ub ADP-ribosylation mediated by the Parp9 catalytic domain.
(A) Parp9 mutations engineered in the macrodomains (G112, 311E) and catalytic domain (Δ796-819; Δ684-687) expressed in E. coli and tested for Ub ADP-ribosylation (ADPr-biotin) and heterodimerization with Dtx3L. The locations of the mutations are depicted within a structural model of Parp9 (Figure S4).
(B) Parp9 mutations engineered in the catalytic domain (Y702A; P767A, E768A; F703K) expressed in mammalian cells and tested for Ub ADP-ribosylation (ADPr-biotin) and heterodimerization with Dtx3L.
(C, D) E3 activity of WT Parp9 and F703K Parp9 using recombinant heterodimers with Dtx3L.
(E) Effect of non-selective Parp inhibitors Menadione and Novobiocin on Ub ADP-ribosylation.
(F) Time course of Ub-ADP-ribosylation and H2A ubiquitylation in the presence of Novobiocin.