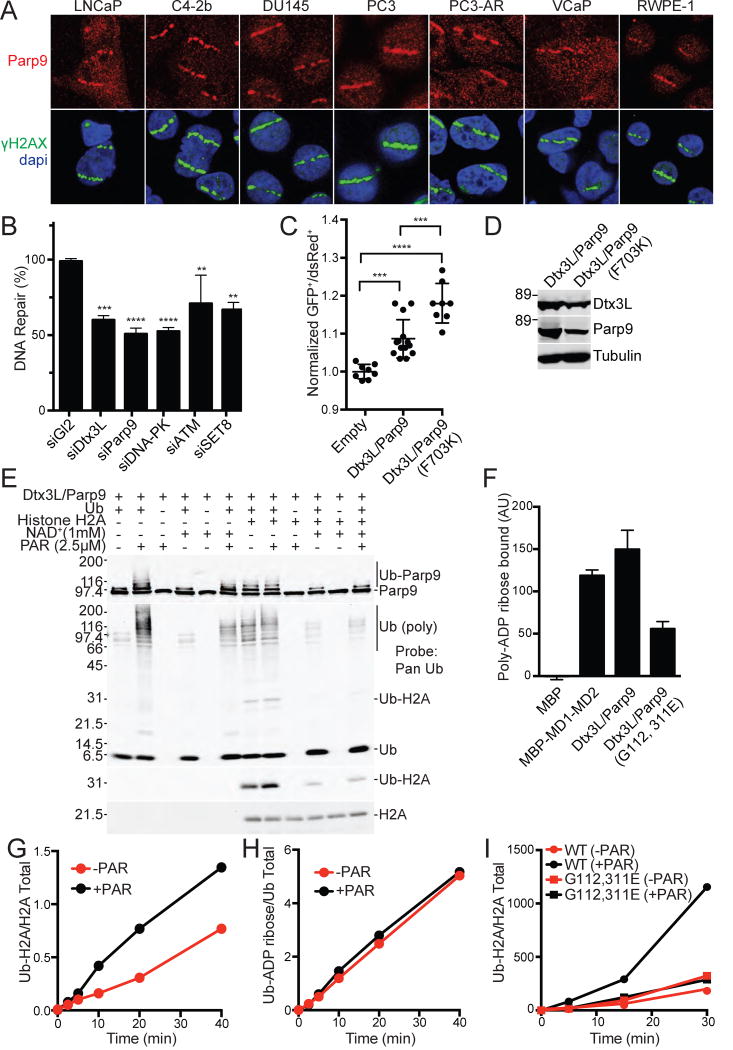
Figure 7. DNA repair and PAR binding activities of Dtx3L/Parp9.
(A) Immunofluorescent localization of endogenous Dtx3L/Parp9 heterodimer to microirradiated DNA stripes.
(B) Depletion of Dtx3L/Parp9 by siRNA reduces DNA repair by NHEJ. Depletion of DNA PK, ATM, and SET8 were performed in parallel as positive controls. DNA repair was measured in 293T cells containing an integrated reporter that undergoes Cas9/sgRNA mediated cleavage and subsequent repair by NHEJ. Bar graphs are represented as the mean and SD. **P≤0.01, ***P≤0.001, P≤0.0001.
(C) The DNA repair function of Dtx3L/Parp9 is increased in a loss-of-function Parp9 catalytic domain mutant (F703K). DNA repair by NHEJ was assayed using a plasmid-based reporter. Data points are pooled from three experiments, and the mean and SD for each condition are indicated.
(D) Expression levels of Dtx3L/Parp9 proteins in the plasmid-based reporter assay (in Figure 7C).
(E) PAR stimulates the E3 activity of the Dtx3L/Parp9 heterodimer.
(F) PAR binding to Dtx3L/Parp9 heterodimer is reduced by single point mutations in each of the macrodomains. Proteins were spotted on nitrocellulose, incubated with biotin-tagged PAR, and binding detected with FL-Neutra (Figure S7).
(G, H) PAR stimulates ubiquitylation of Histone H2A without ADP-ribosylation of Ub (blots in Figure S7E).
(I) PAR enhancement of histone ubiquitylation is lost upon mutation of the Parp9 macrodomains (blots in Figure S7F).