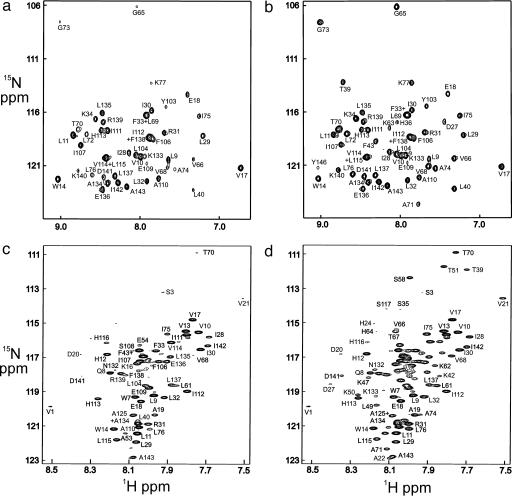
Fig. 3.
HSQC spectra of myoglobin refolded with various folding times in the quench-flow experiment. (a) Urea-jump experiment measured using the folded holoprotein in D2O solution with a folding time of 6.4 ms. (b) Urea-jump experiment using the folded holoprotein in D2O solution with a folding time of 3 s. (c) pH-jump experiment measured in DMSO solution using the unfolded apoprotein with a folding time of 6.4 ms. (d) pH-jump experiment measured in DMSO solution using the unfolded apoprotein with a folding time of 3 s. The upfield region of the spectrum, containing the glycine cross peaks, has been omitted from c and d, due to extremely low intensities.