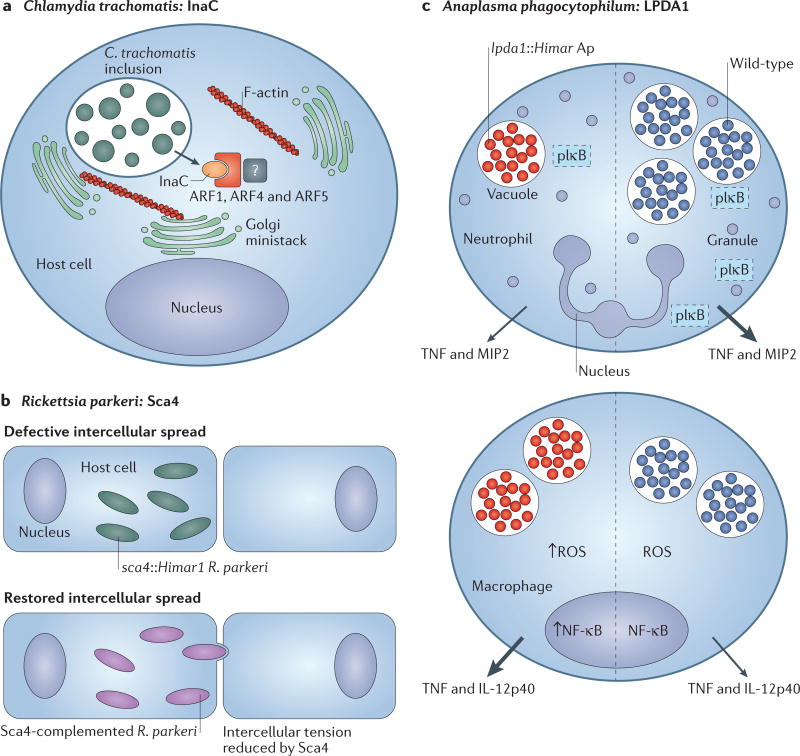
Figure 4. Discoveries in microbial pathogenesis facilitated by genetic tools.
a Chemical mutagenesis of Chlamydia trachomatis led to the identification of inclusion membrane protein for actin assembly (InaC), which recruits f ilamntous actin (F-actin) and induces Golgi redistribution around the inclusion in manner that is dependent on ADP ribosylation factor 1 (ARF1), ARF4 and ARF5 (REF. 8). b | Rickettsia parkeri disseminates intercellularly through a mechanism that is not used by facultative intracellular bacteria. Screening of a Himar1 transposon mutagenesis library of R. parkeri identified a mutant deficient in intercellular spread named surface cell antigen 4 (sca4)Himar1 (REF. 10). The sca4, Himar1 mutant was complemented by pRAM18dRGA[Sca4], which restored intercellular spread. Sca4 was found to inhibit vinculin binding to α-catenin (not shown), thereby reducing intercellular force transduction and enabling the intercellular spread of R. parkeri. c | Himar1 transposon mutagenesis was used to generate an Anaplasma phagocytophilum mutant library. One mutant exhibited a single transposon insertion in the dihydrolipoamide dehydrogenase 1 (lpda1) gene. Compared with wild-type infection, the lpda1, Himar1 A. phagocytophilum mutant infected neutrophils less well, and activated nuclear factor-κB (NF-κB) poorly (as measured by decreased phosphorylated inhibitory subunit of NF-κB (pIκB)), and elicited the production of less tumour necrosis factor (TNF) and macrophage inflammatory protein 2 (MIP2; also known as CXCL2) in neutrophils70. Transient infection of macrophages by the lpda1, Himar1 A. phagocytophilum strain correlated with enhanced nuclear NF-κB activity, which led to the increased production of reactive oxygen species (ROS) and the release of proinflammatory cytokines70. Mice that were infected with the lpda1, Himar1 mutant or wild-type A. phagocytophilum HZ exhibited differences in immunopathology and cell-specific outcomes, which suggests that LPDA1 may have a role in inhibiting excessive host immune activation70. Arrow size correlates with relative quantity of cytokines secreted. IL-12p40, interleukin-12 subunit p40.