Figure 5. The PP-motif is not sufficient to confer the ability to activate TNAP to other ZnT transporters.
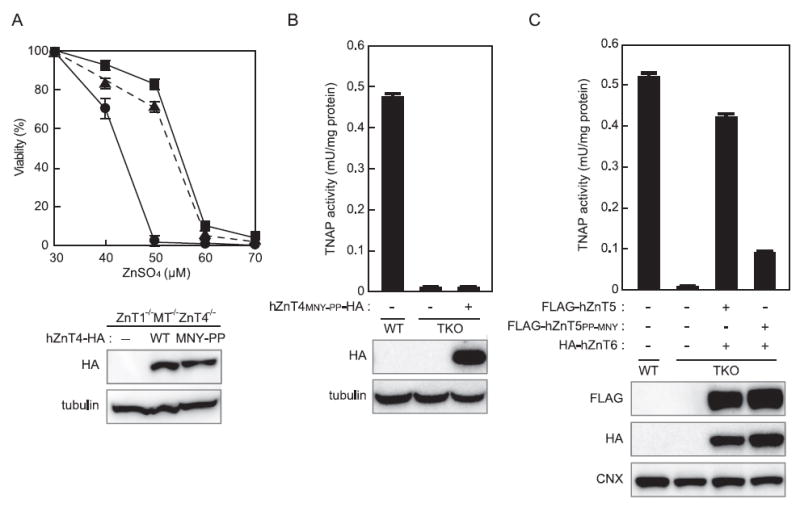
(A) Evaluation of the zinc transport activity of hZnT4 mutants using ZnT1−/− MT−/− ZnT4 −/− cells. ZnT1 −/− MT −/− ZnT4 −/− cells stably expressing WT hZnT4 or the hZnT4MNY-PP mutant were grown in the presence of the indicated concentrations of ZnSO4 for 72 h, and the number of living cells was measured by the Alamar Blue assay (and plotted as a percentage of living cells at 30 μM ZnSO4 for each group of cells). ZnT1−/− MT −/− ZnT4 −/− cells (●) and ZnT1 −/− MT −/− ZnT4 −/− cells stably expressing WT hZnT4 (■) or hZnT4MNY-PP (▲). Each value is the mean ± S.D. for three independent experiments. (B) TNAP activity in TKO cells is not restored by expressing the hZnT4MNY-PP mutant. (C) TNAP activity is significantly reduced in TKO cells co-expressing the hZnT5PP-MNY mutant with hZnT6. In (B) and (C), TNAP activity is expressed as the mean ± S.D. for three independent experiments. The expression of each ZnT protein was confirmed by immunoblotting (lower panels). Tubulin and calnexin (CNX) are shown as loading controls.
