Fig. 1. Characterization of single intraperitoneal AAV9 vector administration in neonatal female PCK rats.
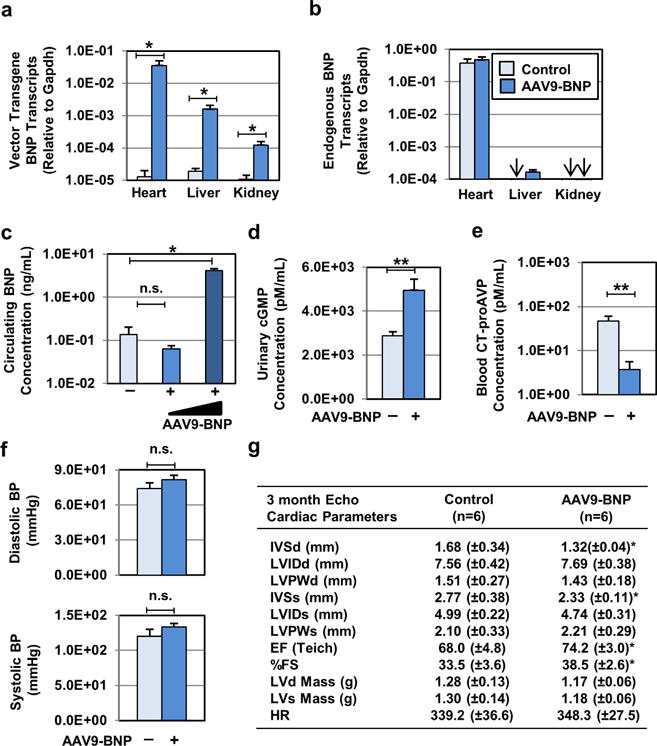
(a) Biodistribution of AAV9 vector upon neonatal AAV9 vector administration was monitored. Codon-optimized vector transgene BNP and (b) endogenous BNP transcript levels in the heart, kidney, and liver samples were determined by RT-PCR, followed by normalization to Gapdh transcript levels. Control (n=8) and AAV9-BNP vector-treated (n=6, 1.0×1013 vg/kg) PCK rats were used. Arrows indicate where transcripts assayed were below detection. (c) Circulating levels of BNP from littermate control PCK (n=6), AAV9-BNP (n=9, 1.0×1013 vg/kg) and High-Dose AAV9-BNP (n=5, 1.0×1014 vg/kg) were determined by rat BNP45 ELISA. (d) Urinary excreted cyclic GMP (cGMP) and (e) plasma levels of CT-proAVP from control (n=10) and AAV9-BNP (n=10, 1.0×1013 vg/kg) were determined. (f) Noninvasive diastolic and systolic blood pressure measurements were determined in AAV9-BNP-treated (n=15, 1.0×1013 vg/kg) and control littermate PCK rats (n=10). Both groups remained normotensive 3 months post treatment. (g) Echocardiographic assessment (Echo) of cardiac structure and function was performed in PCK (n=7) and AAV9-BNP-treated (n=10, 1.0×1013 vg/kg) groups. Improved cardiac function and reduced cardiac mass were associated with AAV9-BNP vector administration. Intraventricular septum, diastole (IVSd), Left ventricular internal diameter, diastole (LVIDd), Left ventricular posterior wall, diastole (LVPWd), Intraventricular septum, systole (IVSs), Left ventricular internal diameter, systole (LVIDs), Left ventricular posterior wall, systole (LVPWs), Ejection Fraction (EFteich), Percent fractional shortening (%FS), Left ventricular Mass, diastole (LVd Mass), Left ventricular mass, Systole (LVs Mass). *P < 0.05 and **P < 0.01, vs. PCK by t test. Data represent the mean ± SEM.
