Fig. 4.
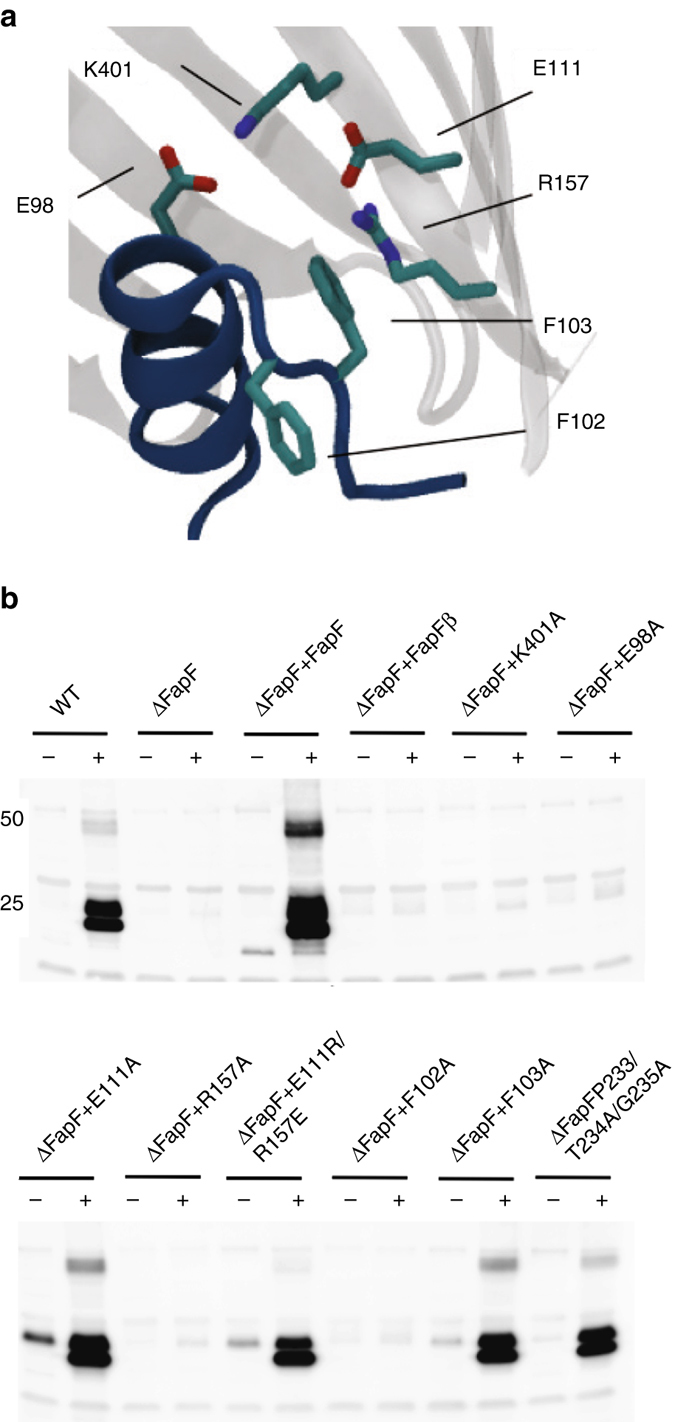
Heterologous FapC amyloid production in E. coli. a Position of residues assessed in this work within the crystal structure. b Shown are secretion assays for the ∆fapF operon complemented with full-length, wild-type FapF, ∆fapF operon complemented with FapFβ and various point mutations of FapF within the operon itself. These were treated with either fomic acid (+) or water (−). If FapC is secreted to form amyloid, it is detected by ɑFapC as a band at ~23 kDa after formic acid treatment. A second band is also produced of slight lower molecular weight which likely reflects a degradation product. Approximate MW markers of 50 and 25 kDa are shown as indicated. Full, uncropped gels are shown in Supplementary Fig. 13
