Figure 1.
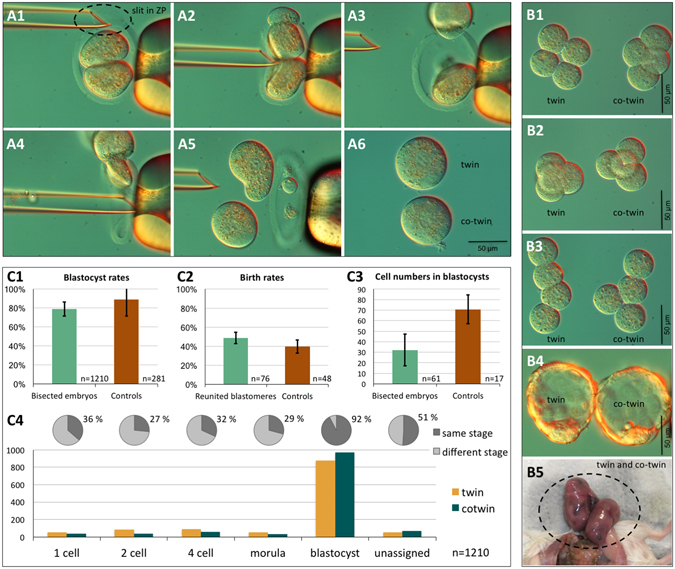
Micromanipulation and culture of two-cell mouse embryos to generate monozygotic (MZ) twins. Embryos were held in a micromanipulation medium lacking calcium and phosphate. A holding pipette (right side in the picture of series A) applied negative pressure to hold a two-cell stage embryo at the equatorial region, aligning its cleavage axis with the pipette’s long axis. Using a spiked beveled pipette as a bisection tool (left side in the picture), a slit was made in the zona pellucida (ZP) at the upper pole of the embryo (A1). The upper blastomere was pushed through the slit by gently pressing the bisection pipette against the ZP at the equator, while the pressure in the holding pipette was slightly reduced (A2–A3). The second blastomere was released by pressing the ZP below the equator (A4–A5). Separated blastomeres recovered their round shape within a few minutes after bisection (A6). Upon culture in KSOM(aa) medium, the sister blastomeres cleaved and developed to the four-cell stage (planar conformation, (B1); tetrahedral conformation, (B2); open chain conformation, B3) and further to the blastocyst stage (B4). When MZ blastocysts were transferred as a pair to the same uterine horn, cases were recorded in which two fetuses were found in that horn (B5), suggesting that at least some two-cell embryos were totipotent in both blastomeres. Monozygotic twins formed blastocysts at the same rate (C1), comprising half the number of cells as unmanipulated blastocysts (C3). After transfer to the uterus, blastocysts obtained from bisected and then immediately reunited blastomeres yielded as many fetuses as unmanipulated controls (C2). The error bars associated with the diagram bars are standard deviations. Twin pairs that formed two blastocysts were equally frequent among the sister blastomeres, as were twin pairs that arrested at pre-blastocyst stages (C4).
