Figure 1.
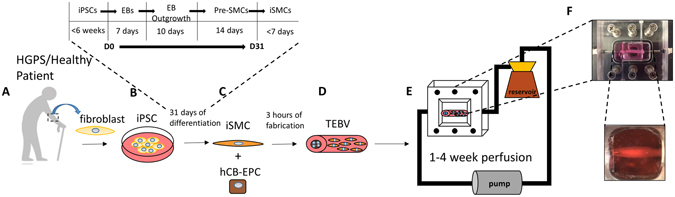
Schematic diagram of the procedure to produce iPSC-derived SMC TEBVs from healthy and HGPS patients. (A) A fibroblast biopsy from either healthy (young or old) or HGPS individuals (B) is converted to induced pluripotent stem cell (iPSC) cultures. (C) iPSCs are then differentiated into induced smooth muscle cells (iSMCs) using a 31-day process as previously described by Xie et al.21. (D) iSMCs are then incorporated into a dense collagen gel construct that is seeded with human cord blood-derived endothelial cells from a separate, healthy donor on the luminal surface to create iSMC TEBVs using the process previously described by Fernandez et al.20. (E) iSMC TEBVs are then incorporated into a flow loop and perfused with steady laminar flow at a shear stress of 6.8 dynes/cm2 for 1 to 4 weeks for maturation and functional characterization studies. (F) Photographic images of HGPS iSMC TEBVs in the custom perfusion chambers under perfusion conditions.
