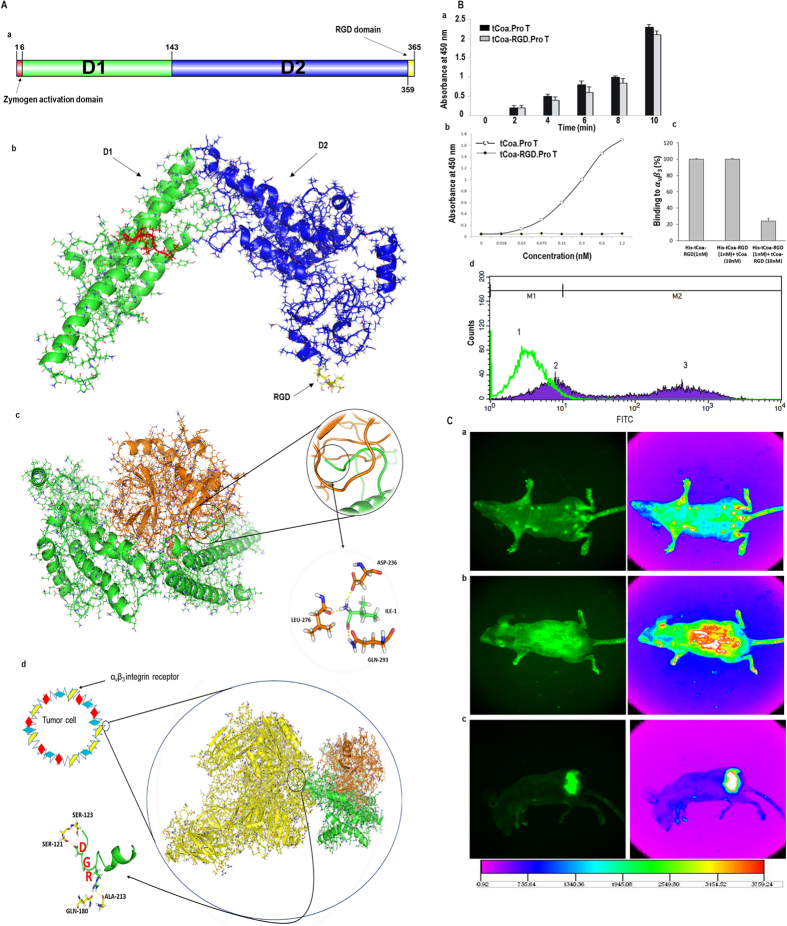
Figure 2.
Functional studies of tCoa-RGD fusion proteins. (A) Modeling, docking, and MD simulation. (a) Amino acid sequence of tCoa-RGD, its domains, and 3D structure. (b) tCoa-RGD has a helical structure within its two domains. (c) The 3D structure of tCoa-RGD in complex with prothrombin after equilibration in MD simulation. Insertion of tCoa-N-terminal into prothrombin is highlighted by a circle. IVTKDY hexapeptide at the N-terminus of protein interacts strongly with zymogen activation domain of prothrombin in a “molecular sexuality mechanism.” (d) The 3D structure of tCoa-RGD-prothrombin in complex with the extracellular domain of αvβ3 integrins after equilibration in MD simulation. The interaction of RGD domain with integrin residues is highlighted with a circle. (B) Functional studies to determine coagulase activity of the fusion proteins (a), and retention of antibody activity by tCoa-RGD to αvβ3 integrins by ELISA (b, c) and FACS (d). (a) Retention of coagulase activity was determined by the capability of tCoa and tCoa-RGD to convert fibrinogen into fibrin through the conformational activation of ProT. tCoa-RGD presented coagulase activity comparable to that of tCoa at each time point, whereas ProT alone did not potentiate coagulation and there was no detectable absorbance. (b) In vitro binding studies demonstrated that tCoa-RGD specifically bind to immobilized αvβ3 integrins in a concentration dependent manner, with the highest binding at 1.2 nM concentration. (c) In presence of His-tag removed tCoa-RGD but not tCoa, binding of tCoa-RGD to the αvβ3 integrins was significantly inhibited (>70%). (d) FACS analysis verified differential binding of tCoa (peck 1) and tCoa-RGD (peck 2, 3) on endothelial cells in suspension. In this histogram, M1 marker covers the negative cells presenting no/none-specific binding while M2 marker comprises the positive cells. Accordingly, specific binding of tCoa-RGD to the αvβ3 integrins was determined 64.24% (peck 3). None-specific binding for tCoa and tCoa-RGD was measured 98% and 36.05%, respectively. Correspondingly, the measured fluorescence intensity for tCoa-RGD was eight times higher than that of tCoa. (C) Tracing of fluorescently labeled drugs in vivo. tCoa-RGD labeled with FITC injected to (a) healthy mice with no tumor; while (b) tCoa and (c) tCoa-RGD injected to mice bearing SKOV3 ovarian carcinoma xenografts. tCoa-RGD fusion protein but not tCoa showed specific accumulation at the subcutaneously implanted tumor site in C57Bl/6 nude mice.