ABSTRACT
Organelles such as neuropeptide-containing dense-core vesicles (DCVs) and mitochondria travel down axons to supply synaptic boutons. DCV distribution among en passant boutons in small axonal arbors is mediated by circulation with bidirectional capture. However, it is not known how organelles are distributed in extensive arbors associated with mammalian dopamine neuron vulnerability, and with volume transmission and neuromodulation by monoamines and neuropeptides. Therefore, we studied presynaptic organelle distribution in Drosophila octopamine neurons that innervate ∼20 muscles with ∼1500 boutons. Unlike in smaller arbors, distal boutons in these arbors contain fewer DCVs and mitochondria, although active zones are present. Absence of vesicle circulation is evident by proximal nascent DCV delivery, limited impact of retrograde transport and older distal DCVs. Traffic studies show that DCV axonal transport and synaptic capture are not scaled for extensive innervation, thus limiting distal delivery. Activity-induced synaptic endocytosis and synaptic neuropeptide release are also reduced distally. We propose that limits in organelle transport and synaptic capture compromise distal synapse maintenance and function in extensive axonal arbors, thereby affecting development, plasticity and vulnerability to neurodegenerative disease.
KEY WORDS: Dense-core vesicle, Secretory granule, Mitochondria, Axonal transport, En passant boutons, Neuropeptide
Summary: Synaptic function and supply of organelles such as vesicles and mitochondria diminish in distal monoaminergic axon terminals.
INTRODUCTION
Synaptic transmission relies on axonal transport of organelles such as dense-core vesicles (DCVs), small synaptic vesicle (SSV) precursors and mitochondria to sequential varicose release sites in axonal arbors called en passant synaptic boutons. Recently, it was shown that glutamatergic type Ib en passant boutons at the Drosophila neuromuscular junction (NMJ) are supplied equivalently with functional DCVs by vesicle circulation and bidirectional capture (Wong et al., 2012, 2015). Furthermore, synaptic capture of DCVs increases following activity and is constitutively elevated by a transcription factor in specialized neuroendocrine neurons (Shakiryanova et al., 2006; Bulgari et al., 2014; Cavolo et al., 2016). Disease-related proteins such as the fragile-X syndrome protein and huntingtin also influence synaptic neuropeptide stores by regulating direction-specific DCV capture (Cavolo et al., 2016; Bulgari et al., 2017). Thus, synaptic capture is a major determinant of presynaptic accumulation of DCVs and hence affects the capacity for release of DCV contents (e.g. neuropeptides and neurotrophins).
Vesicle circulation was discovered in small arbors that innervate one or two targets with a limited number of boutons. However, it is not known if this mechanism is responsible for organelle distribution in neurons with many axonal branches projecting to multiple postsynaptic targets, each with numerous en passant boutons. Such extensive fields of innervation are common for neurons that release neuromodulators such as neuropeptides and monoamine transmitters. For example, a single substantia nigra dopamine neuron axon forms >100,000 synapses in rats and millions of synapses in humans (Matsuda et al., 2009; Bolam and Pissadaki, 2012). Interestingly, it has been proposed that the metabolic load of supporting such extensive innervation for volume transmission contributes to the vulnerability of substantia nigra dopamine neurons in Parkinson's disease (Matsuda et al., 2009; Bolam and Pissadaki, 2012; Pacelli et al., 2015). Yet, it is not known whether numerous, widely distributed synaptic boutons are efficiently supplied with organelles by transport from a single axon. For circulation to distribute organelles equally to many boutons in an extensive arbor, either capture at proximal en passant boutons would have to be scaled down or axonal transport would have to be scaled up.
Determining the routing of organelles to synaptic boutons in extensive fields of innervation in the intact mammalian brain is not technically feasible, but can be approached by studying synaptic organelle traffic in identified neurons of a simpler nervous system. Therefore, we studied innervation by larval Drosophila neurons that release the stress-associated monoamine octopamine, which may be packaged in both DCVs and SSVs (Grygoruk et al., 2014). Specifically, we focused on larval neurons that each innervate ∼20 dorsal muscles with axonal branches that are hundreds of micrometers long and can feature ∼100 type II boutons per muscle (Monastirioti et al., 1995; Hoang and Chiba, 2001; Koon et al., 2011). The large arbor with numerous boutons from a single octopamine neuron contrasts with excitatory type Ib glutamatergic NMJs, which typically form much smaller arbors on a single muscle with far fewer boutons (e.g. ∼5–25 on muscles 13 and 4). This difference in innervation is easily understood given that glutamate directly elicits electrical excitation, while octopamine regulates basal and evoked larval muscle contractility (Ormerod et al., 2013) and development of glutamatergic synapses even on muscles lacking type II innervation (Koon et al., 2011). Thus, identified Drosophila octopamine neurons offer the opportunity to elucidate how organelles are distributed in an extensive field of monoaminergic innervation from a single axon associated with neurohormone action reminiscent of monoaminergic volume transmission in the mammalian brain.
Here, genetic manipulations and imaging show that octopamine neuron axonal arbors feature distal boutons with fewer DCVs and mitochondria. In vivo imaging shows that DCV transport and synaptic capture are not scaled to support vesicle circulation. Furthermore, stimulated synaptic neuropeptide release and endocytosis are attenuated in distal boutons. These results suggest that distal synaptic function in extensive axonal arbors operates at limits imposed by organelle transport and synaptic capture.
RESULTS
Neuropeptide content and synaptic function are decreased in distal type II boutons
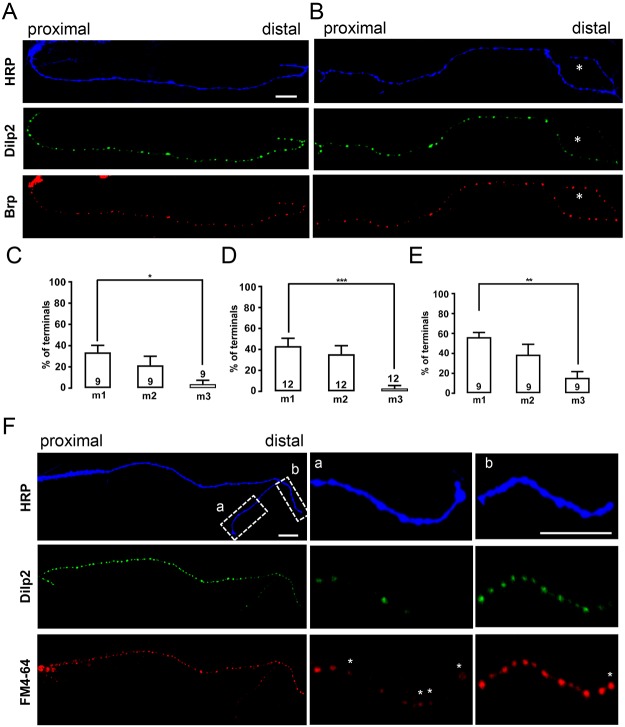
We began our studies by examining the abundance of emerald GFP (green fluorescent protein)-tagged neuropeptides in peripheral octopamine neuron axon arbors, which contain type II boutons. Specifically, we focused on a single segmental octopamine neuron that innervates ∼20 dorsal muscles including muscles 1–3 (m1–3) (Hoang and Chiba, 2001), which are in the same layer and numbered by distance from the soma in the central nervous system (i.e. with m1 being most distal, Fig. S1A). Data were obtained with constructs based on Drosophila insulin-like peptide 2 (Dilp2) (Wong et al., 2012), which produces the brightest signal, and rat atrial natriuretic factor (ANF) (Rao et al., 2001) to ensure that results applied to multiple neuropeptides. Importantly, such GFP constructs report native neuropeptide abundance and behavior-associated release in Drosophila and so are validated as selective DCV markers (Rao et al., 2001; Husain and Ewer, 2004; Loveall and Deitcher, 2010). Expression was driven specifically in octopamine neurons with the Tdc2–Gal4 driver, while expression was produced in other terminals of interest with the pan-neuronal driver elav–Gal4. Neuropeptide signals were compared with neuronal membrane immunofluorescence labeled with TRITC- or Alexa-405-conjugated anti-horseradish peroxidase (HRP) antibody; please note that unless indicated otherwise, all images are presented with distal boutons on the right with proximal and distal indicated (or distal denoted by d), and defects are highlighted with an asterisk. Examination of octopaminergic innervation with anti-HRP revealed that each muscle has ∼80 type II boutons (Fig. S1). Although proximal boutons reliably contained DCVs (Fig. 1A,B and Fig. S2A,B, left), some distal sites labeled with anti-HRP contained less signal from labeled DCVs (Fig. 1B and Fig. S2A,B, right). This distal deficiency (indicated with asterisks in Fig. 1 and Fig. S2) was most prevalent in the muscles that were furthest from the CNS (m1, Fig. S2C); specifically, the percentage of terminals with lower neuropeptide content in distal boutons was statistically different between the most distal muscle (m1) and a more proximal muscle (m3) regardless of the driver or the GFP-labeled peptide (Fig. 1C-E).
Fig. 1.
Neuropeptide stores, active zones and endocytosis in distal type II boutons. (A) Neuronal membrane (HRP), Dilp2–GFP and Brp in type II boutons on muscle m1. All images are oriented with distal boutons on the right. Dilp2–GFP expression was driven by Tdc2–Gal4 and terminals are labeled by TRITC–HRP immunofluorescence. In this example, DCVs and active zones are widely distributed in boutons. (B) Similar experiment to that in A, except that the most-distal boutons (marked with asterisks) contain less Dilp2–GFP, although Brp is present. (C–E) Quantification of the percentage of axonal branches on the indicated muscles in Tdc2>Dilp2-GFP animals (C), Tdc2>ANF-GFP animals (D) or elav>ANF-GFP animals (E) lacking neuropeptide content in distal boutons.The number of examined neurons, each from an independent animal is indicated for each muscle. For each neuron, all the branches on each muscle were imaged and the number of branches with dimmer distal boutons was divided by the total number of branches to calculate the percentage for each muscle. One-way ANOVA with Tukey's post-test was performed to determine statistical significance. *P<0.05, **P<0.01, ***P<0.001. (F) Comparison of Dilp2–GFP and uptake of the SSV marker FM4-64 on m2. Asterisks indicate boutons with FM4-64 that lack Dilp2–GFP. Scale bars: 10 µm.
Distal HRP labeling without DCVs might be attributed to retraction resulting in some residual membrane, but not intact organelle-containing boutons. However, several experimental observations exclude this possibility. First, although distal boutons can be completely lacking in detectable DCVs, adjustment of gain and contrast showed that the GFP signal is often present, but dimmer, than in proximal boutons (Fig. S2C). This implies that distal HRP labeling can arise from DCV-containing boutons, albeit with less total neuropeptide content. Second, immunofluorescence showed that the active zone marker Bruchpilot (Brp) is present in distal type II boutons regardless of the abundance of the DCV marker (Fig. 1A,B, bottom). The presence of active zones suggests that distal HRP-labeled endings are intact boutons. Third, we measured endocytosis of the styryl dye FM4-64 when acutely evoked by depolarization for minutes. Importantly, no FM4-64 labeling was induced in Ca2+-free medium, verifying detection of activity-evoked synaptic endocytosis. However, depolarization in the presence of Ca2+ showed that FM4-64 labeling can be evident in boutons even when neuropeptide-containing DCVs are not readily apparent (Fig. 1F, asterisks mark FM4-64 labeling at sites without Dilp2–GFP). Fourth, mitochondria were present in some distal boutons without DCVs (see below). Finally, as shown below, traffic experiments reveal a difference in nascent DCV transport even away from the most distal type II boutons. Therefore, multiple lines of evidence show that retraction does not account for the paucity of neuropeptide in distal boutons.
We then compared the function of proximal to distal boutons. In each experiment, a data point was obtained by averaging the responses of 7 or 8 boutons in each region (i.e. proximal and distal). First, we quantified depolarization-evoked Ca2+-dependent endocytosis of FM4-64, which does not correlate with DCVs (Fig. 1F). This uptake was attenuated in distal boutons compared with the most proximal boutons in a branch (Fig. 2A). Next, we considered whether diminished neuropeptide content of distal boutons is a consequence of higher DCV-mediated release by distal boutons. Therefore, we compared neuropeptide release from DCVs in distal and proximal boutons induced by depolarization (i.e. by measuring the loss of Dilp2–GFP fluorescence). In contrast to distal type Ib boutons, which show no difference in release among boutons (Shakiryanova et al., 2006; Wong et al., 2015), the percentage of peptide released from type II boutons evoked by depolarization is ∼50% lower for distal boutons (Fig. 2C). Therefore, less distal peptide cannot be attributed to enhanced release. Rather, distal boutons in extensive type II arbors are less capable of peptide release because they tend to have fewer DCVs and peptide release for the DCVs that are present is less efficient. Hence, two aspects of stimulated synaptic function, neuropeptide release and endocytosis, are attenuated in distal type II boutons.
Fig. 2.
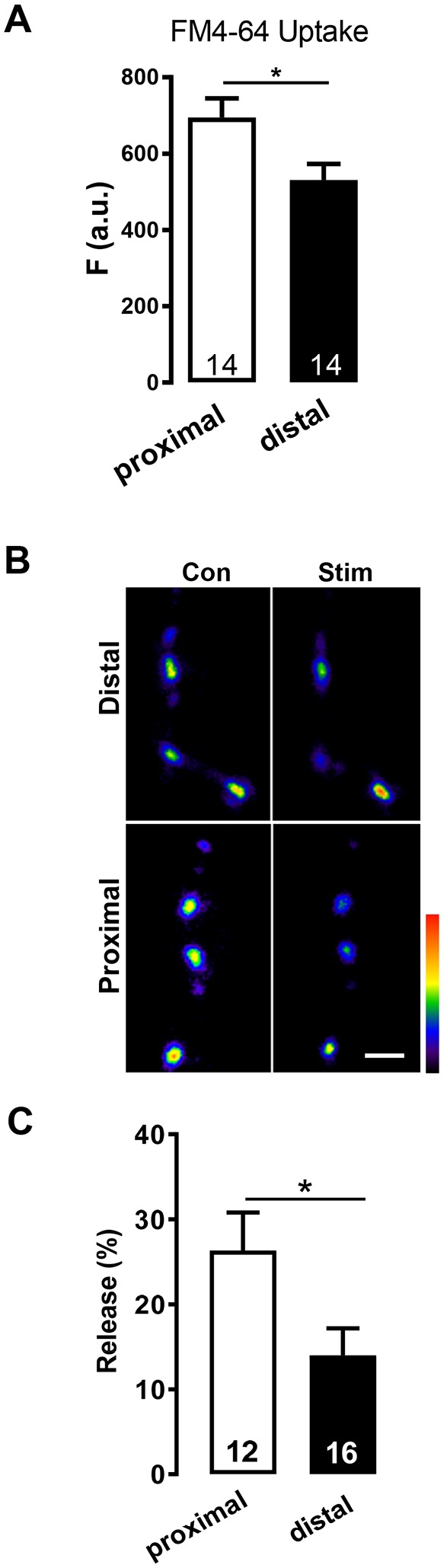
Decreased endocytosis and neuropeptide release at distal type II boutons. (A) Quantification of FM4-64 uptake in the proximal region (i.e. within the 7 to 8 boutons closest to the axon) and the distal region (within the 7 to 8 boutons from the axon terminus) on m2. N is the number of regions, which were from 12 animals. F, fluorescence; a.u., arbitrary units. (B) Pseudo-color images of Dilp2–GFP in distal (top) and proximal (bottom) type II boutons on m2 before (Con) and after stimulation by depolarization for 3 min (Stim). Scale bar: 2 µm. (C) Quantification of percentage release in proximal and distal boutons calculated as loss of GFP fluorescence. Total number of boutons examined in 4 animals is indicated for each group. *P<0.05, Student's t-test.
DCV transport and capture in type II arbors
These results raised the possibility that accumulation of DCVs in type II boutons occurs differently than in type Ib boutons, where circulation to and from the most distal bouton with bidirectional capture along the way leads to equivalent delivery and neuropeptide release (Wong et al., 2012, 2015). To test this hypothesis, we used approaches that previously revealed vesicle circulation in type Ib boutons. First, we examined the age of DCVs in proximal and distal type II boutons by imaging a timer construct (ANF tagged with monomeric Kusabira Green Orange, mK-GO, driven by 386Y-Gal4) that converts from green to red fluorescence over hours (Tsuboi et al., 2010; Bulgari et al., 2014). Fluorescence red to green ratio (Fred/green) showed that, in contrast to findings in type Ib boutons that are supplied by vesicle circulation, DCVs in distal type II boutons are older than their proximal cohort (Fig. 3A,B).
Fig. 3.
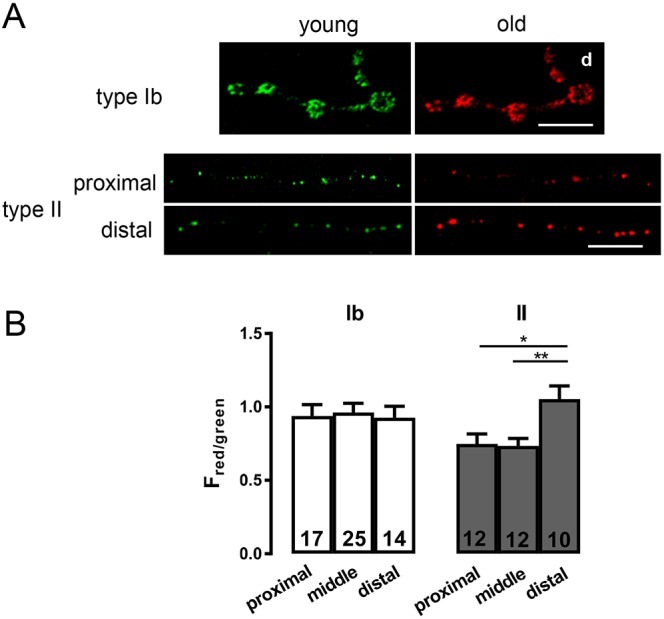
DCV age differs in proximal and distal boutons. (A) Age-dependent labeling of DCVs containing ANF tagged with the mK-GO timer protein driven by 386Y-Gal4 in type Ib and type II boutons on m2. The green (young) and red (old) images are shown side by side. Scale bars: 10 µm. For type Ib image, the distal boutons are on the right (labeled d), while separate images are shown for proximal and distal regions containing type II boutons. (B) The ratio of red to green fluorescence in type Ib and type II boutons. Number of terminals analyzed in 12 animals is indicated for each region and bouton type. One-way ANOVA and Tukey's post-tests were performed to determine statistical significance of differences between red to green ratios in proximal, middle and distal regions containing 7 to 8 boutons each. **P<0.01, *P<0.05.
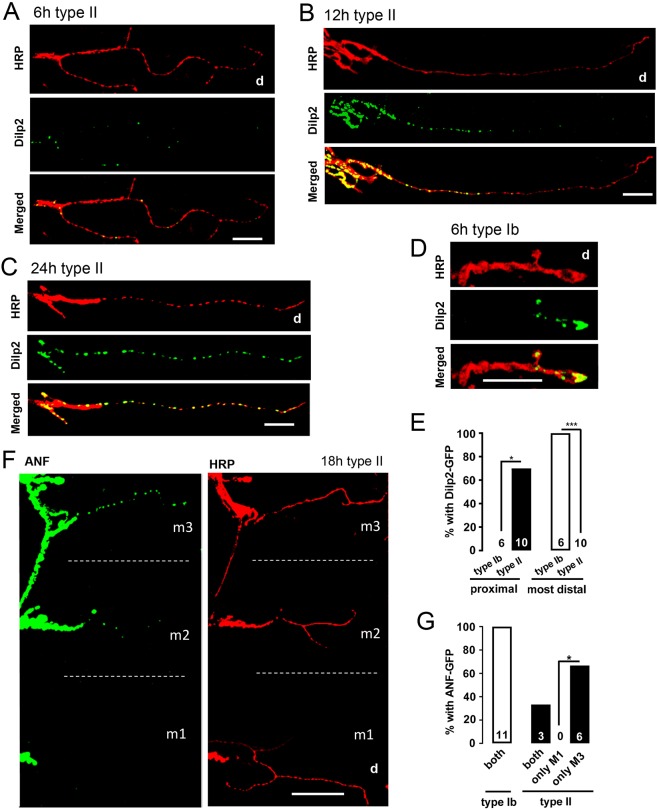
To follow up on this result, we determined how newly synthesized peptide is distributed in type II arbors by inducing GFP-tagged neuropeptide synthesis. With GeneSwitch drivers GS65605 and GS3550-2 (Nicholson et al., 2008), peptide accumulation appeared first in proximal type II boutons and distal accumulation was only detected much later (Fig. 4A–C and Fig. S3). This contrasts with the initial accumulation of GeneSwitch-induced DCVs in distal type Ib boutons (Fig. 4D,E) (Wong et al., 2012). Furthermore, type II boutons on a muscle closer to the octopamine neuron cell bodies in the central nervous system (m3) were supplied with nascent DCVs more efficiently than a more distal muscle (m1) (Fig. 4F); in these experiments m3 type II boutons always contained newly made GeneSwitch-induced DCVs while m1 type II boutons had a detectable signal in only a third of the cases (Fig. 4G). Thus, nascent DCV delivery is biased proximally on a single muscle and between multiple muscle targets of the same axon.
Fig. 4.
DCVs accumulate initially in proximal type II boutons. Third instar larvae were fed RU486 for different periods of time. TRITC-conjugated HRP antibody labels the neuronal membrane. Boutons on m2 are shown. Representative images of Dilp2–GFP in type II terminals of the GS65606 line after RU486 induction for 6 h (A), 12 h (B) and 24 h (C). Scale bars: 25 µm. Distal regions are indicated (d). (D) Image of Dilp2–GFP in a type Ib terminal after RU486 treatment for 6 h. Scale bar: 10 µm. (E) Quantification of the percentage of type Ib and type II NMJs with Dilp2–GFP after 6 h RU486 treatment in proximal and distal boutons. Number of type Ib or II endings examined in the experiment, which were obtained from 6 animals for type Ib and 10 animals for type II, is indicated for each group; 0% appears as no bar. Fisher's exact test was performed to calculate the P-values. *P<0.05, ***P<0.001. (F) Representative images of ANF–GFP distribution in type II boutons driven by GS3550-2 after RU486 induction for 18 h on different muscles. The dotted line distinguishes different muscles. Scale bar: 100 µm. (G) Quantification of the ANF–GFP distribution in type Ib and II boutons driven by GS3550-2 with 18 h RU486 treatment on different muscles (both, both m1 and m3; only m1, m1 but not m3; only m3, m3 but not m1). Data are derived from 11 animals for type Ib and 9 animals for type II. Hypothesis testing for proportions was used to analyze the statistical significance between different groups. *P<0.05; d, distal.
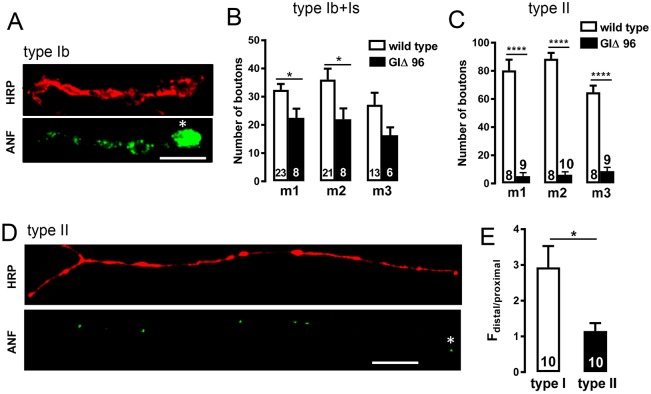
We then examined the effect of inhibiting retrograde transport by expressing a dominant-negative Glued subunit (also known as DCTN1) of the dynactin complex (i.e. with UAS-GlΔ96) (Allen et al., 1999). In type Ib boutons, inhibition of retrograde transport results in distal accumulation of DCVs because they cannot leave the distal bouton to circulate back into proximal boutons (Fig. 5A, asterisk indicates distal bouton) (Wong et al., 2012). The effectiveness of GlΔ96 expression in octopamine neurons was evident from the marked reduction in number of type II boutons in arbors on m1–3 (Fig. 5B,C) that was revealed with anti-HRP labeling. However, in the presence of UAS-GlΔ96, there was no distal bias in accumulation of peptide in type II boutons (Fig. 5D,E). Thus, retrograde transport is important for development of type II arbors. However, in contrast to vesicle circulation to populate type Ib boutons, retrograde transport is not important for populating type II boutons with DCVs.
Fig. 5.
Retrograde transport is not required for DCV distribution in type II terminals. (A) ANF–GFP with expression of a dominant-negative form of the dynactin Glued subunit (UAS-GlΔ96) in type Ib processes on m2 labeled with elav–GAL4. The membrane is labeled with TRITC-HRP antibody. Asterisk indicates the most distal bouton. Scale bar: 10 µm. (B,C) Quantification of the total number of boutons of type I (Ib+Is) (B) and type II (C) terminals on each muscle in the wild type and in GlΔ96 overexpression larvae based on anti-HRP labeling. The number on each bar represents how many endings were analyzed from 8 animals for type I boutons and 10 animals for type II boutons. Student's t-test was used to calculate statistical significance; *P<0.05, ****P<0.0001. (D) ANF–GFP in type II boutons on m2 with expression of GlΔ96. The asterisk indicates the most distal bouton. Scale bar: 10 µm. (E) Quantification of the ratio of fluorescence intensity of distal boutons to proximal boutons. Total number of m2 endings examined derived from 10 animals is shown for each group. Student's t-test was used to calculate the P-value. *P<0.05.
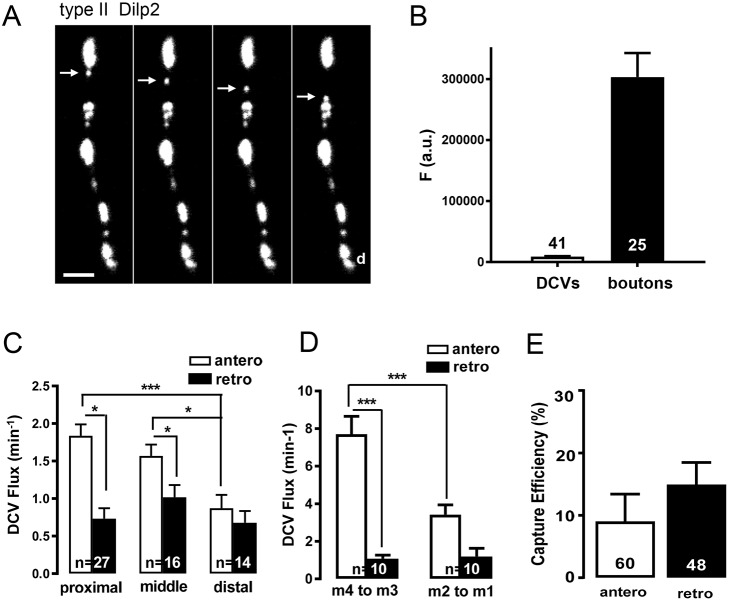
The inefficient anterograde delivery to distal boutons in extensive type II arbors led us to quantify DCV transport. First, we imaged boutons and individual puncta traveling between boutons (Fig. 6A), the latter of which are likely to be single DCVs (Shakiryanova et al., 2006; Scalettar et al., 2014). Because DCVs and whole type II boutons are smaller than the depth of field of our optics, the ratio of fluorescence between boutons and puncta (Fig. 6B) can be used to estimate that there are ∼34 DCVs per bouton. Furthermore, anterograde DCV flux at the most proximal boutons on m3 was about half that previously found in type Ib boutons (Fig. 6C, Shakiryanova et al., 2006). This difference is relatively modest compared with the dramatic difference in innervation in the two neuron subtypes. However, in contrast to type Ib boutons in which anterograde and retrograde flux are balanced, but in accordance with the limited impact of inhibiting retrograde transport revealed with GlΔ96, there is relatively little retrograde DCV flux in proximal type II boutons. This trend is also evident in the axon upstream of m3 (Fig. 6D). Interestingly, anterograde flux and the imbalance with retrograde flux are diminished as DCVs progress to distal boutons (Fig. 6C) and axonal branches (i.e. axons between m2 and m1) (Fig. 6D). This result is consistent with DCVs, which usually travel efficiently through type Ib boutons (Shakiryanova et al., 2006; Wong et al., 2012), being recruited by type II boutons along the way.
Fig. 6.
DCV transport and capture in type II boutons. (A) Time-lapse imaging (2 Hz) of Dilp2–GFP-labeled DCVs in a fillet. Arrow indicates punctum moving by anterograde transport (toward distal boutons). Scale bars: 2 µm. Note that the nonsaturated image data are contrast enhanced to show puncta. (B) Fluorescence of type II boutons (n=25) and DCVs between type II boutons (n=41). Data from m2 in 3 animals. (C) Anterograde (open bars) and retrograde (filled bars) DCV flux at proximal, middle and distal type II boutons. Numbers on the bars in the graph indicate the number of endings analyzed from 38 animals. Data are derived from m1-m3. One-way ANOVA with Tukey's post-test compared anterograde or retrograde transport between three different regions. *P<0.05, ***P<0.001. (D) DCV anterograde and retrograde flux in the axonal regions projecting from m4 to m3 and from m2 to m1. Number of axonal regions analyzed from 10 animals is shown for each group. One-way ANOVA with Tukey's post-test was performed to determine statistical significance; ***P<0.001. (E) DCV capture efficiency in type II boutons. Total number of boutons from m1-m3 analyzed from a total of 38 animals is shown.
This process has been termed capture and has been measured as the decrease in DCV flux entering and leaving a bouton (Shakiryanova et al., 2006; Wong et al., 2012; Bulgari et al., 2014, 2017; Cavolo et al., 2016). Notably, this parameter encompasses capture initiation, which when measured over minutes does not quantify the much longer duration of capture (Shakiryanova et al., 2006). Nevertheless, the onset of capture (i.e. the mismatch in flux entering and leaving a bouton on the time scale of minutes) is altered by activity, a peptidergic neuron transcription factor and Huntingtin to regulate synaptic neuropeptide stores (Wong et al., 2012; Bulgari et al., 2014, 2017; Cavolo et al., 2016). Thus, this parameter is limiting in the supply of DCVs to synaptic boutons. In type Ib boutons, capture efficiency is ∼10% in each direction (Wong et al., 2012). Type II boutons display similar capture efficiency (Fig. 6E). Given the large number of en passant type II boutons distributed on a large axonal arbor, DCVs are therefore unlikely to reach the most distal boutons because flux will be depleted by branching and capture en route.
Mitochondrial distribution and synaptic neuropeptide release
We then considered whether the difficulty in supplying distal type II boutons with DCVs applies to mitochondria. Mitochondrial labeling with GFP or DsRed was driven with the type II specific driver Tdc2–Gal4 or elav–GAL4. First, we noted that, in contrast to type Ib terminals (Fig. 7A), but reminiscent of mammalian terminals (Nafstad and Blackstad, 1966; Shepherd and Harris, 1998), not all proximal type II boutons contain mitochondria (Fig. 7B). Mitochondria in en passant boutons of Drosophila motoneurons facilitate sustained release from SSVs (Guo et al., 2005; Verstreken et al., 2005). To explore whether this function might extend to DCVs in type II boutons, we measured depolarization-evoked GFP-tagged neuropeptide release from strings of proximal type II boutons in which Mito–DsRed revealed varying presence of mitochondria. These experiments, which were based on quantification of loss of GFP fluorescence in boutons possessing or lacking a Mito–DsRed signal, showed that depolarization-evoked neuropeptide release from DCVs was 2.5-fold greater in boutons with mitochondria (Fig. 7C). Although these data do not imply a causal relationship, they show that the presence of mitochondria correlates with greater synaptic release from DCVs.
Fig. 7.
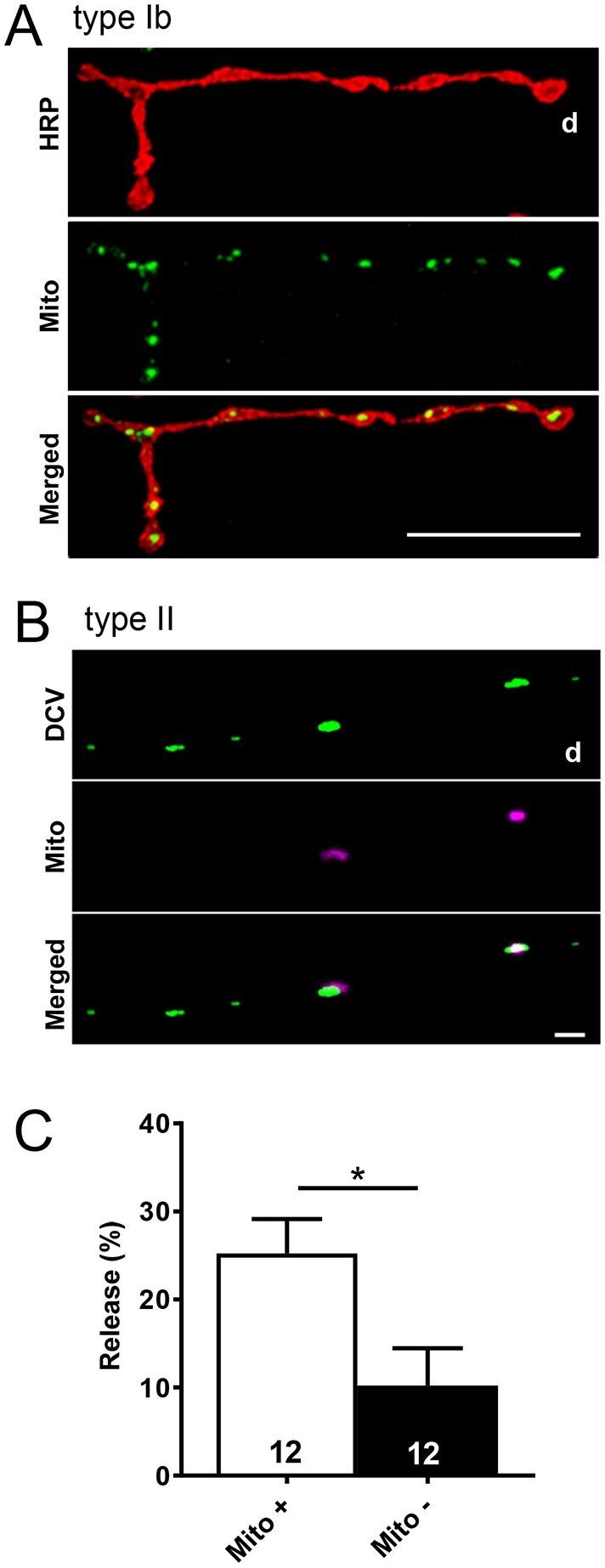
Mitochondria and neuropeptide release in proximal boutons. (A) Type Ib boutons contain mitochondria. Mito–GFP expression on m2 was driven by OK6–GAL4. TRITC-HRP labeling of the neuronal membrane is also shown. Scale bar: 20 µm. (B) Proximal type II boutons on m2 do not always contain mitochondria. Mito–DsRed and Dilp2–GFP expression were driven by Tdc2–GAL4. Scale bar: 2 µm. (C) Quantification of depolarization-evoked synaptic peptide release, measured as loss of GFP, at the proximal type II boutons with mitochondria (Mito +) and without mitochondria (Mito −), as indicated by Mito–DsRed labeling. Total number of boutons examined in 4 animals is indicated. *P<0.05, Student's t-test.
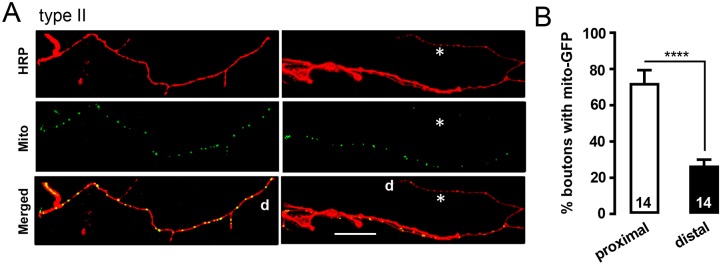
This led us to determine the prevalence of mitochondria in proximal and distal type II boutons. As was found with DCVs in type II terminals, distal ends varied in the prevalence of mitochondria; Fig. 8A, left shows an example in which distal mitochondria are evident, while Fig. 8A, right is an example of distal boutons lacking in mitochondria (asterisks indicate regions with distal deficiency). Quantification verified that the probability that mitochondria were in distal boutons was significantly less than in proximal boutons (Fig. 8B). Thus, the absence of distal mitochondria may contribute to the lower efficiency of distal neuropeptide release and endocytosis that occurs as part of the SSV cycle (Fig. 2), which is known to be affected by mitochondria under high demand conditions (Rangaraju et al., 2014; Pathak et al., 2015; Sobieski et al., 2017). Together, the results presented here demonstrate deficient accumulation of two types of organelles – DCVs and mitochondria – and attenuated synaptic function at the most distal sites of extensive monoaminergic innervation.
Fig. 8.
Mitochondria are often absent from distal type II boutons. (A) Type II terminals on m1 expressing mito–GFP driven by Tdc2–GAL4. The left panels show an example with mitochondria widely distributed in a type II process, whereas the right panel shows a process in which mitochondria are absent distally. Asterisk indicates area with no mitochondria; d, distal region. HRP immunofluorescence was used to stain the terminals. Scale bar: 20 µm. (B) Quantification of the percentage of boutons with mitochondria out of the proximal eight and distal eight boutons of type II terminals. Total number of processes examined in 11 animals is indicated for each group. ****P<0.0001, Student's t-test.
DISCUSSION
Organelles in distal type II boutons
Neurons must support distant synaptic release sites by the axonal transport of organelles. However, it was unclear how organelles undergoing anterograde transport are apportioned among en passant boutons until it was discovered that neuropeptides are delivered by DCV capture during vesicle circulation between the proximal axon and the most distal boutons (Wong et al., 2012). Because this mechanism was studied in small axonal arbors with few type Ib boutons, we hypothesized that much larger axonal arbors with many more boutons could also be supplied by vesicle circulation if axonal transport and/or capture were scaled to match innervation. However, the current study of octopamine neurons demonstrated that distal type II boutons are not efficiently supplied with DCVs and mitochondria. Furthermore, traffic experiments in octopamine neuron axonal arbors showed that retrograde traffic is more limited, DCVs are not preferentially transported to the most distal boutons and distal DCVs are older than their proximal counterparts. These findings all contrast with findings in type Ib boutons and indicate that type II boutons are not supplied by vesicle circulation.
Why does distal organelle accumulation differ between neuron subtypes? In type II boutons, capture efficiency is comparable to type Ib boutons, but innervation is far more extensive, with a single axon possessing ∼1500 boutons distributed on ∼20 targets. Therefore, the greater number of en passant boutons in type II arbors has dramatic consequences. For example, with 10% capture in 4 en passant boutons, only 34% of the DCVs are captured before reaching the most distal bouton, where the remaining 66% accumulate before reversing direction to undergo retrograde transport. This is reminiscent of the rapid delivery of neuropeptides in distal type Ib boutons as part of vesicle circulation (Wong et al., 2012). However, with similar capture efficiency in 26 en passant type II boutons, only 6.5% of the DCVs will reach the most distal bouton without being captured en route (i.e. 10-fold less than in type Ib boutons). Consistent with this explanation, the bias against delivery to distal type II boutons is not evident when the number of boutons is reduced by the dominant negative Glued (Fig. 5). Notably, the proximal bias in delivery that accompanies many en passant boutons could have been overcome by increasing the DCV axonal transport, but DCV flux failed to reveal such compensation. Therefore, the differences in distal DCV accumulation and function between type Ib and II boutons are accounted for by a lack of scaling of transport and capture to preserve vesicle circulation according to the number of boutons supported by each axon.
The shared problem in supplying distal boutons with DCVs and mitochondria was not expected because these organelles rely on different anterograde axonal transport motors. Specifically, transport of mitochondria requires kinesin-1 (Kif5 in mammals) whereas transport of neuropeptide-containing DCVs and synaptic vesicle proteins requires unc-104 (Kif1A in mammals) (Jacob and Kaplan, 2003; Pack-Chung et al., 2007; Hirokawa et al., 2010). Indeed, the independence of axonal transport of DCVs and mitochondria is evident in proximal type II boutons, which often are devoid of mitochondria while containing DCVs (Fig. 7B). Nevertheless, the distal absence of both organelles in type II terminals implies that transport by the two motors shares a common limitation. It is possible that synaptic capture of cargos of both motors is not scaled down sufficiently to allow cargo-carrying motors to travel through scores of boutons to reach the most distal points of octopamine neuron innervation. Alternatively, because DCV transport is attenuated, but not eliminated, by loss of kinesin-1 (Djagaeva et al., 2012), the involvement of kinesin-1 in the transport of both mitochondria and DCV provides a potential link that could explain their common problem with distal accumulation. However, because the absence of mitochondria and DCVs is not always coincident in individual boutons, there must not be a tight coupling between the mechanisms governing distribution of these organelles in distal boutons.
Function of distal type II boutons
Strikingly, the absence of delivery by organelle circulation is accompanied with less neuropeptide release and endocytosis. Thus, inefficient organelle delivery is associated with inefficient synaptic function at the furthest reaches of extensive octopaminergic innervation. For neuropeptide release, the impact of fewer DCVs is apparent from previous experiments, which show that release scales with presynaptic neuropeptide stores (e.g. Bulgari et al., 2014). Ultrastructural studies confirm that rapid stimulation-induced Ca2+-dependent FM dye uptake into Drosophila boutons is mediated by endocytosis to form SSVs (e.g. Denker et al., 2009; Akberenova and Bykhovskaia, 2009). Thus, the observed attenuated FM labeling at distal type II boutons is suggestive of less SSV cycling, which is expected to reduce synaptic transmission. Interestingly, endocytosis for SSV recycling and ‘kiss-and-run’ exocytosis for synaptic neuropeptide release both involve dynamin (Holz, 2013; Wong et al., 2015). Furthermore, in part because of dynamin, SSV recycling is a major consumer of ATP, which is provided by mitochondria when transmission is intense (Rangaraju et al., 2014; Pathak et al., 2015; Sobieski et al., 2017). Therefore, it will be of interest to test the hypothesis that the lower abundance of distal mitochondria limits dynamin function to diminish both endocytosis and neuropeptide release.
Neurons present dual challenges of transport distance and complex morphology that are not encountered in the more compact cells in which organelle transport mechanisms evolved. Therefore, the lack of scaling of organelle delivery for extensive innervation may reflect inherent limits in motors and the synaptic capture mechanism. Accordingly, our study of a monoaminergic neuron supports the proposal that limitations in organelle supply contribute to the association of extensive axonal arbors with vulnerability, as is found with mammalian dopamine neurons of the substantia nigra in Parkinson's disease (Matsuda et al., 2009; Bolam and Pissadaki, 2012; Pacelli et al., 2015). It is also notable that the early stages of motoneuron degeneration are associated with compromised distal function. Again, function may be linked to organelle abundance at distal endings. Finally, the inefficient supply of organelles to distal boutons in extensive arbors may impact development, retraction and plasticity of the synapse. Therefore, diminished organelle delivery to distal sites of extensive innervation may be a general feature of neuronal cell biology that impacts synaptic function under physiological and pathological conditions. We suggest that neurons push the limits of transport and synaptic capture to support as much innervation as possible when this is advantageous. Apparently, for volume transmission that is associated with neuromodulation by monoamines and neuropeptides, it is worthwhile living with suboptimal distal function as a consequence of cell biology to maximize the number and distribution of transmission sites.
MATERIALS AND METHODS
Drosophila
Transgenic flies used to visualize vesicles in both type I and II boutons or in type II boutons specifically were elav>ANF-GFP (yw, elav-Gal4, UAS-preproANF-EMD) (Rao et al., 2001), Tdc2>ANF-GFP (UAS-preproANF-EMD; Tdc2-Gal4) (Grygoruk et al., 2014; kindly provided by David Krantz, UCLA), Tdc2>Dilp2-GFP (Tdc2-Gal4, UAS-Dilp2-GFP/CyO) and 386>mK-GO (w; +/+; 386Y-Gal4, UAS-Timer mK-GO/TM3, Ser). Third instar larvae were studied in imaging experiments; females were selected with the first two lines, while both sexes were used in the latter line. Gene induction experiments employed transgenic GeneSwitch (GS) lines, which were kindly provided by Haig Keshishian (Yale University) or obtained from the Bloomington Stock Center. Specifically, female larvae of a stable recombinant line GS3550-2>ANF-GFP were used or GS65605/CyO male flies were crossed with female UAS-Dilp2-GFP/CyO flies and F1 larvae of either sex were used. To study the role of retrograde transport, male wild type (Canton S) or UAS-Glued GlΔ96 (Allen et al., 1999) were crossed with female elav>ANF-GFP flies and F1 male larvae were selected. To visualize the distribution of mitochondria, male UAS-mito-GFP (Pilling et al., 2006; Bloomington 8443) flies were crossed to female Tdc2-Gal4 or OK6-Gal4 flies. For imaging neuropeptide release and mitochondria, male w; UAS-mito-DsRed flies (Lutas et al., 2012; kindly provided by Fumiko Kawasaki, Pennsylvania State University) were crossed to female Tdc2>UAS-Dilp2-GFP flies and F1 larvae were used for imaging.
All animals were raised in incubators at 25°C. For RU486 GeneSwitch induction, GS>ANF-GFP or GS>Dilp2-GFP larvae were transferred to food containing 100 µg/ml RU486 (stock: 100 mg/ml in ethanol solution, Mifepristone; Sigma-Aldrich). Freshly cooked Jazzmix food (Thermo Fisher Scientific) was cooled to <60°C before being mixed with the RU486 stock. In time course experiments, at different time points (6 h, 12 h, 18 h, 24 h), larvae at 25°C were removed from the food with RU486 and filleted.
Imaging
The third or fourth hemi-segment from the head was examined for each animal. Filleted larvae were imaged in Ca2+-free HL3 solution (70 mM NaCl, 5 mM KCl, 0.5 mM Na3-EGTA, 20 mM MgCl2, 10 mM NaHCO3, 5 mM trehalose, 115 mM sucrose and 5 mM HEPES, pH 7.2) on an upright Olympus Fluoview 1000 confocal microscope with a 20× (NA 0.95) or a 60× (NA 1.10) dipping water immersion objective. Laser excitation at 405, 488 and 561 nm was used to image fluorescent proteins and conjugated anti-HRP antibodies. In timer (mK-GO) experiments, excitation was switched between 488 and 561 nm laser with simultaneous switching between a green band-pass and red long-pass filter. DCV flux was imaged on the confocal microscope through the cuticle in intact anesthetized larvae. Specifically, Tdc2>Dilp2-GFP larvae were exposed to isoflurane vapor for 5 to 7 min and then were then transferred to a slide with a drop of HL3 solution (i.e. same solution as above except with EGTA replaced with 1.5 mM CaCl2). A coverslip (#1.5) then squeezed the larvae flat with its dorsal side up. The slide was then viewed with a 60× (NA 1.45) or 20× (NA 0.85 NA) oil immersion objective to image DCVs at 0.5 Hz at a single plane of focus. If imaging was disrupted by muscle contractions, the anesthetic was applied again to the same larva for a couple of minutes or a new larva was used. A well-anesthetized larva could then be imaged for 15 to 20 min and fully awakened after 30–60 min of recovery on food. For quantification of DCV and bouton fluorescence, imaging was performed on spinning disk microscope equipped with a 14-bit EM-CCD camera.
For stimulation of synaptic neuropeptide release, larvae expressing Dilp2-GFP with or without UAS-mito-DsRed were filleted in Ca2+-free HL3 medium and then depolarized by application of High K+ HL3 (i.e. same as HL3 except that 70 mM NaCl was replaced with KCl) for 3 min. Synaptic release, measured as the loss of GFP-tagged neuropeptide fluorescence, was imaged after the medium was replaced with Ca2+-free HL3 as previously described (Levitan et al., 2007) with quantification of intensity carried out with ImageJ software (http://rsb.info.nih.gov/ij/). Flux was determined by manually counting DCVs moving through a region of interest (ROI) (i.e. between boutons) over a period of minutes. For fluorescence intensity measurements, the ROI (e.g. surrounding a bouton) in one frame was selected; then another ROI was drawn away from boutons for background subtraction.
Immunofluorescence and FM4-64
For labeling of neuronal membranes, live fillets were stained with anti-HRP (Jackson ImmunoResearch) antibody conjugated with either TRITC (lot 120714, 1:100 dilution) or Alexa Fluor 405 (lot 124382, 1:300 dilution) for 15 min. The bathing solution was then replaced and organelles imaged as described above. For Brp labeling, filleted larvae were fixed with 4% paraformaldehyde for 30 min and washed three times for 15 min each in PBST (PBS with 0.1% Triton X-100) at room temperature. After blocking the non-specific binding with a block solution (PBST+5% goat serum, Sigma-Aldrich) for 20 min, the animals were incubated in dark with the primary antibody (mouse anti-Brp NC82; 1:100, Developmental Studies Hybridoma Bank) overnight at 4°C, then with the secondary antibody (goat anti-mouse IgG Alexa568, lot 1069849, 1:250, Invitrogen) for 4.5 h at room temperature. Unbound antibodies were removed by washing with PBST three times for 15 min each at room temperature. Finally, samples were mounted in Fluoroshield mounting medium (Sigma-Aldrich) with #1.5 coverslips for imaging with a confocal microscope.
FM4-64 dye (Invitrogen, lot 1785953) was dissolved in water to make a 1 mM stock, which was stored at 4°C. FM4-64 was then diluted 100-fold in High K+ HL3 to yield a final concentration of 10 µM. Fillets were bathed in this solution for 4.5 min and then washed five times with Ca2+-free HL3 for 3 min. Imaging was performed with water immersion objectives as described above.
Statistics
Error bars show standard error of the mean (s.e.m.). Two-tailed P-values for Student's t-test, Fisher's exact text, one way ANOVA and Tukey's post-test were determined in GraphPad Prism, while hypothesis testing for proportions (Fig. 3G) was performed by the Statistics Consulting Center of the University of Pittsburgh.
Acknowledgements
We thank Fumiko Kawasaki (Pennsylvania State University) for UAS-mito-DsRed flies and David Krantz (UCLA) for Tdc2>ANF-GFP flies. Deposited in PMC for release after 12 months.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: E.S.L.; Formal analysis: J.T., D.B., E.S.L.; Investigation: J.T., D.B.; Resources: D.L.D.; Writing – original draft: E.S.L.; Writing – review and editing: J.T., D.B., D.L.D., E.S.L.; Supervision: E.S.L.; Project administration: E.S.L.; Funding acquisition: E.S.L.
Funding
This research was supported by National Institutes of Health grants R01NS032385 and R21DA038384 to E.S.L. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.201111.supplemental
References
- Akbergenova Y. and Bykhovskaia M. (2009). Stimulation-induced formation of the reserve pool of vesicles in Drosophila motor boutons. J. Neurophysiol. 101, 2423-2433. https://doi.org/10.1152/jn.91122.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. J., Shan X., Caruccio P., Froggett S. J., Moffat K. G. and Murphey R. K. (1999). Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J. Neurosci. 19, 9374-9384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam J. P. and Pissadaki E. K. (2012). Living on the edge with too many mouths to feed: why dopamine neurons die. Mov. Disord. 27, 1478-1483. 10.1002/mds.25135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgari D., Zhou C., Hewes R. S., Deitcher D. L. and Levitan E. S. (2014). Vesicle capture, not delivery, scales up neuropeptide storage in neuroendocrine terminals. Proc. Natl. Acad. Sci. USA 111, 3597-3601. 10.1073/pnas.1322170111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgari D., Deitcher D. L. and Levitan E. S. (2017). Loss of Huntingtin stimulates capture of retrograde dense-core vesicles to increase synaptic neuropeptide stores. Eur. J. Cell Biol. [EPub] 10.1016/j.ejcb.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavolo S. L., Bulgari D., Deitcher D. L. and Levitan E. S. (2016). Activity induces Fmr1-sensitive synaptic capture of anterograde circulating neuropeptide vesicles. J. Neurosci. 36, 11781-11787. 10.1523/JNEUROSCI.2212-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A., Krohnert K. J. and Rizzoli S. O. (2009). Revisiting synaptic vesicle pool localization in the Drosophila neuromuscular junction. J. Physiol. 587, 2919-2926. 10.1113/jphysiol.2009.170985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djagaeva I., Rose D. J., Lim A., Venter C. E., Brendza K. M., Moua P. and Saxton W. M. (2012). Three routes to suppression of the neurodegenerative phenotypes caused by kinesin heavy chain mutations. Genetics 192, 173-183. 10.1534/genetics.112.140798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygoruk A., Chen A., Martin C. A., Lawal H. O., Fei H., Gutierrez G., Biedermann T., Najibi R., Hadi R., Chouhan A. K. et al. (2014). The redistribution of Drosophila vesicular monoamine transporter mutants from synaptic vesicles to large dense-core vesicles impairs amine-dependent behaviors. J. Neurosci. 34, 6924-6937. 10.1523/JNEUROSCI.0694-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Macleod G. T., Wellington A., Hu F., Panchumarthi S., Schoenfield M., Marin L., Charlton M. P., Atwood H. L. and Zinsmaier K. E. (2005). The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 47, 379-393. 10.1016/j.neuron.2005.06.027 [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Niwa S. and Tanaka Y. (2010). Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68, 610-638. 10.1016/j.neuron.2010.09.039 [DOI] [PubMed] [Google Scholar]
- Hoang B. and Chiba A. (2001). Single-cell analysis of Drosophila larval neuromuscular synapses. Dev. Biol. 229, 55-70. 10.1006/dbio.2000.9983 [DOI] [PubMed] [Google Scholar]
- Holz R. W. (2013). Dynamin flexibility drives fission. Science 339, 1392-1393. 10.1126/science.1236005 [DOI] [PubMed] [Google Scholar]
- Husain Q. M. and Ewer J. (2004). Use of targetable gfp-tagged neuropeptide for visualizing neuropeptide release following execution of a behavior. J. Neurobiol. 59, 181-191. 10.1002/neu.10309 [DOI] [PubMed] [Google Scholar]
- Jacob T. C. and Kaplan J. M. (2003). The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 23, 2122-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koon A. C., Ashley J., Barria R., DasGupta S., Brain R., Waddell S., Alkema M. J. and Budnik V. (2011). Autoregulatory and paracrine control of synaptic and behavioral plasticity by octopaminergic signaling. Nat. Neurosci. 14, 190-199. 10.1038/nn.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan E. S., Lanni F. and Shakiryanova D. (2007). In vivo imaging of vesicle motion and release at the Drosophila neuromuscular junction. Nat. Protoc. 2, 1117-1125. 10.1038/nprot.2007.142 [DOI] [PubMed] [Google Scholar]
- Loveall B. J. and Deitcher D. L. (2010). The essential role of bursicon during Drosophila development. BMC Dev. Biol. 10, 92 10.1186/1471-213X-10-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutas A., Wahlmark C. J., Acharjee S. and Kawasaki F. (2012). Genetic analysis in Drosophila reveals a role for the mitochondrial protein p32 in synaptic transmission. G3 (Bethesda) 2, 59-69. 10.1534/g3.111.001586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W., Furuta T., Nakamura K. C., Hioki H., Fujiyama F., Arai R. and Kaneko T. (2009). Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 29, 444-453. 10.1523/JNEUROSCI.4029-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M., Gorczyca M., Rapus J., Eckert M., White K. and Budnik V. (1995). Octopamine immunoreactivity in the fruit fly Drosophila melanogaster. J. Comp. Neurol. 356, 275-287. 10.1002/cne.903560210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafstad P. H. J. and Blackstad T. W. (1966). Distribution of mitochondria in pyramidal cells and boutons in hippocampal cortex. Z. Zellforsch Mikrosk. Anat. 73, 234-245. 10.1007/BF00334866 [DOI] [PubMed] [Google Scholar]
- Nicholson L., Singh G. K., Osterwalder T., Roman G. W., Davis R. L. and Keshishian H. (2008). Spatial and temporal control of gene expression in Drosophila using the inducible GeneSwitch GAL4 system. I. Screen for larval nervous system drivers. Genetics 178, 215-234. 10.1534/genetics.107.081968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod K. G., Hadden J. K., Deady L. D., Mercier A. J. and Krans J. L. (2013). Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae. J. Neurophysiol. 110, 1984-1996. 10.1152/jn.00431.2013 [DOI] [PubMed] [Google Scholar]
- Pacelli C., Giguère N., Bourque M.-J., Lévesque M., Slack R. S. and Trudeau L.-É. (2015). Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr. Biol. 25, 2349-2360. 10.1016/j.cub.2015.07.050 [DOI] [PubMed] [Google Scholar]
- Pack-Chung E., Kurshan P. T., Dickman D. K. and Schwarz T. L. (2007). A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat. Neurosci. 10, 980-989. 10.1038/nn1936 [DOI] [PubMed] [Google Scholar]
- Pathak D., Shields L. Y., Mendelsohn B. A., Haddad D., Lin W., Gerencser A. A., Kim H., Brand M. D., Edwards R. H. and Nakamura K. (2015). The role of mitochondrially derived ATP in synaptic vesicle recycling. J. Biol. Chem. 290, 22325-22336. 10.1074/jbc.M115.656405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling A. D., Horiuchi D., Lively C. M. and Saxton W. M. (2006). Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 17, 2057-2068. 10.1091/mbc.E05-06-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju V., Calloway N. and Ryan T. A. (2014). Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825-835. 10.1016/j.cell.2013.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S., Lang C., Levitan E. S. and Deitcher D. L. (2001). Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 49, 159-172. 10.1002/neu.1072 [DOI] [PubMed] [Google Scholar]
- Scalettar B. A., Shaver D., Kaech S. and Lochner J. E. (2014). Super-resolution imaging of neuronal dense-core vesicles. J. Vis. Exp. 89, 51394 10.3791/51394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D., Tully A. and Levitan E. S. (2006). Activity-dependent synaptic capture of transiting peptidergic vesicles. Nat. Neurosci. 9, 896-900. 10.1038/nn1719 [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. and Harris K. M. (1998). Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 18, 8300-8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieski C., Fitzpatrick M. J. and Mennerick S. J. (2017). Differential presynaptic ATP supply for basal and high-demand transmission. J. Neurosci. 37, 1888-1899. 10.1523/JNEUROSCI.2712-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T., Kitaguchi T., Karasawa S., Fukuda M. and Miyawaki A. (2010). Age-dependent preferential dense-core vesicle exocytosis in neuroendocrine cells revealed by newly developed monomeric fluorescent timer protein. Mol. Biol. Cell 21, 87-94. 10.1091/mbc.E09-08-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P., Ly C. V., Venken K. J. T., Koh T.-W., Zhou Y. and Bellen H. J. (2005). Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, 365-378. 10.1016/j.neuron.2005.06.018 [DOI] [PubMed] [Google Scholar]
- Wong M. Y., Zhou C., Shakiryanova D., Lloyd T. E., Deitcher D. L. and Levitan E. S. (2012). Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell 148, 1029-1038. 10.1016/j.cell.2011.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. Y., Cavolo S. L. and Levitan E. S. (2015). Synaptic neuropeptide release by dynamin-dependent partial release from circulating vesicles. Mol. Biol. Cell 26, 2466-2474. 10.1091/mbc.E15-01-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]