Abstract
Aim
We previously observed a complex pattern of differences in white matter (WM) microstructure between preterm-born (PT) and full-term-born (FT) children and adolescents age 9–17 years. The aim of this study was to determine if the same differences exist as early as age 6 years.
Method
We obtained diffusion MRI (dMRI) scans in children born PT at age 6 years (n = 20; 11 males) and FT (n = 38; 14 males), using two scanning protocols: 30 diffusion directions (b = 1000 s/mm2) and 96 diffusion directions (b = 2500 s/mm2). We used deterministic tractography and analyzed fractional anisotropy (FA) along bilateral cerebral WM pathways that demonstrated differences in the older sample.
Results
Compared to the FT group, the PT group showed (1) significantly decreased FA in the uncinate fasciculi and forceps major and (2) significantly increased FA in the right anterior thalamic radiation, inferior fronto-occipital fasciculi, and inferior longitudinal fasciculi. This pattern of group differences resembles findings in the previous study of older PT and FT participants. Group differences were similar across dMRI acquisition protocols.
Interpretation
The underlying neurobiology driving the pattern of PT-FT differences in FA is present as early as age 6 years. Generalization across dMRI acquisition protocols demonstrates the robustness of group differences in FA. Future studies will use quantitative neuroimaging techniques to understand the tissue properties that give rise to this consistent pattern of WM differences after PT birth.
Keywords: Diffusion tensor imaging, White matter, Prematurity, Development, Tractography
Highlights
-
•
White matter properties are different in preterm and full term children at age 6 years.
-
•
Complex PT-FT differences in fractional anisotropy resemble differences found at 9–17 years.
-
•
PT-FT differences in fractional anisotropy generalize across two dMRI protocols.
-
•
PT showed higher fractional anisotropy than FT in specific tracts.
1. Introduction
Cerebral white matter injury after preterm birth is associated with long-term, adverse neurodevelopmental outcomes (Hintz et al., 2015, Allen, 2008, Aylward, 2005). In the past, many children born preterm (PT) had cystic lesions visualized readily on cranial ultrasound and clinical MRI scans (Gupta et al., 2016, Inder et al., 1999a). Advances in neonatal medicine have reduced the rates of cystic injuries, (Gano et al., 2015) but have not eliminated noncystic white matter injury and subsequent white matter dysmaturity (Volpe, 2009, Back et al., 2007). Such injuries are difficult to detect and quantify on cranial ultrasound or conventional (T1 or T2) MRI scans. Non-cystic injuries may lead to impaired brain growth that can be detected with volumetric analyses; however, these techniques are not widely used in clinical settings (Mathur et al., 2010, Inder et al., 1999b, de la Monte et al., 1990).
Over the past 15 years, diffusion magnetic resonance imaging (dMRI) has emerged as the method of choice for detecting and quantifying white matter microstructure in health and illness (Anjari et al., 2007, Arzoumanian et al., 2003). Tractography is an algorithmic approach applied to dMRI data, which is considered a sensitive method for identifying long-range white matter pathways (tracts) in individual participants (Yeatman et al., 2012). Microstructural properties of these tracts can be quantified by calculating fractional anisotropy (FA), a scalar value that indexes the degree of directional preference of water diffusion at each voxel within the tract (Basser and Pierpaoli, 1996). FA can be decomposed into axial diffusivity (AD) and radial diffusivity (RD), which quantify the speed of water diffusion along the principal and perpendicular diffusion directions, respectively (Feldman et al., 2010). Because bundles of axons constrain water diffusion in a directional fashion, water diffusion has greater directional preference within white matter than in cerebral spinal fluid or in gray matter; FA is higher in white matter than in other tissue compartments. FA increases with increased myelination and high axonal density (Basser and Pierpaoli, 1996). FA decreases with larger axonal diameter and increased numbers of crossing fibers within each voxel in the brain (Jeurissen et al., 2013, Assaf and Pasternak, 2008). The value of FA in any given voxel reflects the relative contributions of these neurobiological factors.
Studies have used dMRI tractography in infants born preterm at- and near- term equivalent ages to assess white matter microstructure in the aftermath of PT birth and to explore associations of early white matter properties with neurodevelopmental outcomes (Pannek et al., 2014, Hasegawa et al., 2011, Adams et al., 2010, Liu et al., 2010, Berman et al., 2005). However, few dMRI tractography studies of PT-born neonates had a FT comparison group (Kaur et al., 2014, Ball et al., 2013, Thompson et al., 2011). These studies comparing PT and FT-born neonates find evidence for early impairments in white matter microstructure in PT children: FA was increased in FT compared to PT neonates in the corpus callosum, uncinate fasciculus, inferior fronto-occipital fasciculus and cingulum (Kaur et al., 2014, Thompson et al., 2011). A lack of studies with a longitudinal design make it unclear whether early white matter differences in the newborn period change with experience, or if new differences emerge as a consequence of on-going developmental delays.
Previous evidence has shown that dMRI can be sensitive to microstructural differences in white matter in the absence of gross structural injuries in adolescents born preterm (Travis et al., 2015a, Groeschel et al., 2014, de Kieviet et al., 2014, Inder et al., 1999a). Three dMRI studies used tractography as the analytic method. However, the findings have been inconsistent. One study comparing PT and FT children at age 8 years consistently found decreased FA in the PT group in six tracts: bilateral cingulum hippocampal tracts, bilateral corticospinal tracts, forceps major and forceps minor (de Kieviet et al., 2014). By contrast, a second study of adolescents born PT compared to FT at age 16 years demonstrated mixed findings: decreased FA in segments of the corpus callosum and corticospinal tract, and increased FA in the superior longitudinal fasciculus, thalamo-cortical pathway, anterior thalamic radiation and segments of the corpus callosum (Groeschel et al., 2014). A third study compared PT and FT children at age 9–17 years and found, again, a mixed pattern of results: PT participants had decreased FA in segments of the uncinate fasciculi (UF), forceps major (FMajor) and right inferior fronto-occipital fasciculus (IFOF), and increased FA in segments of the anterior thalamic radiations (ATR), inferior fronto-occipital fasciculi and inferior longitudinal fasciculi (ILF) (Travis et al., 2015a). Though the age range of participants was broad, there was no association between age and FA (Travis et al., 2015a). The differences in findings across these three studies may relate to differences between the samples (e.g., in age composition) or to differences between the scan protocols and analytic approaches applied in each study. In the current study, we set out to examine patterns of differences in the tract properties of PT and FT born children as early as age 6 years, using methods previously applied in PT and FT at age 9–17 years (Travis et al., 2015a).
We hypothesized that FA differences detected at late childhood to adolescence (Travis et al., 2015a) would be detected at age 6 years, based on the lack of associations with age within the older sample. We specifically expected to find decreased FA in PT compared to FT in segments of the UF, FMajor and IFOF, and increased FA in segments of the ATR, IFOF and ILF in the PT compared to the FT sample. We assessed white matter microstructure using two different acquisition protocols to examine the robustness of the findings.
2. Material and methods
2.1. Participants
Participants were enrolled in a longitudinal study investigating the neural basis of reading in children born preterm, for which children were recruited from the San Francisco Bay Area from 2012 to 2015. For the analyses presented in this study, PT birth was defined as gestational age (GA) < 31 weeks and FT birth was defined as GA > 37 weeks. This definition was employed to equate the mean GA of the PT and FT samples to those in Travis et al. (Travis et al., 2015a). Gestational age was used as the estimate of newborn immaturity and not birth weight because methods for estimating gestational age have improved and because birth weight is comprised of both gestational age and intra-uterine growth rate and is therefore less specific for estimating immaturity than gestational age. Only children who completed both dMRI acquisition protocols at age 6 years (see below) were included in the current study. PT children were recruited from the High-Risk Infant Follow Clinic at Lucile Packard Children's Hospital Stanford, local parent groups and surrounding communities. FT children were recruited through online parent groups, postings in local school newsletters and letters to families who had participated in past research studies in affiliated research laboratories at Stanford University. Exclusion criteria for all participants included congenital anomalies, mother's self-reported use of illicit drugs or alcohol during pregnancy, active seizure disorder, hydrocephalus or sensorineural hearing loss. Diagnosis of cerebral palsy (CP) was not an exclusion criterion. One PT participant had mild CP. The experimental protocol was approved by the Stanford University Institutional Review Board #IRB-22233. A parent or legal guardian provided informed written consent and participants were compensated for participation.
Characteristics assessed in this sample included ethnicity and socio-economic status (SES), as measured using a modified 4-Factor Hollingshead Index (Supplementary material). Intellectual abilities (IQ) were assessed using the Wechsler Abbreviated Scale of Intelligence (WASI-II). Handedness was measured using the Edinburgh Handedness Inventory.
2.2. Diffusion MRI acquisition and analyses
MRI data were acquired on a 3 T Discovery MR750 scanner (General Electric Healthcare, Milwaukee, WI, USA) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA) at the Center for Cognitive and Neurobiological Imaging at Stanford University (www.cni.stanford.edu). All subjects were scanned for research purposes and without the use of sedation. For each subject, we collected a high-resolution T1-weighted anatomical image using a 5-min inversion recovery (IR)-prep 3D fast-spoiler gradient (FSPGR) sequence collected in the sagittal plane (0.9-mm cubed voxel size), and two separate diffusion-weighted sequences. Protocol 1 consisted of a 30-direction diffusion-weighted scan (b = 1000 s/mm2) and Protocol 2 consisted of a 96-direction diffusion-weighted scan (b = 2500 s/mm2). Imaging parameters, data preprocessing steps and analysis of motion are described in the Supplementary material for both acquisition protocols (see Supplementary material).
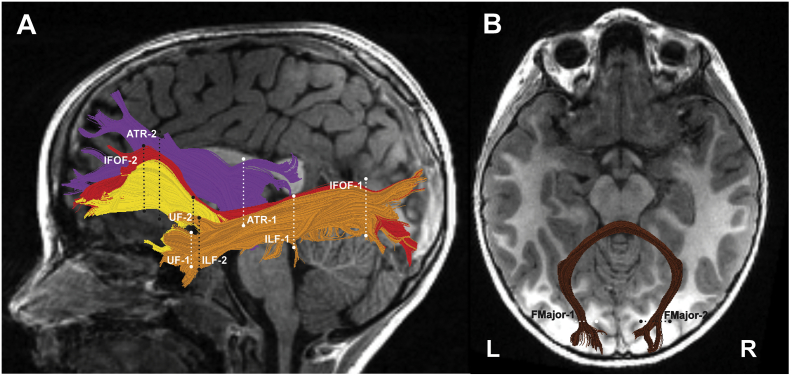
In order to maximize sensitivity while taking into account considerable individual variability, particularly at this early stage in brain development, our approach used individual tract identification in the native space of each child. We also quantified diffusivity properties along the length of the tract rather than rely on less sensitive mean values. Methods for performing deterministic tractography, fiber tract identification, segmentation and quantification were implemented using the Automated Fiber Quantification (AFQ; https://github.jyeatman/AFQ) software package and MATLAB and are described in detail in Supplementary material. These procedures were directly comparable to those employed by Travis et al. (Travis et al., 2015a). Bilateral pathways for analysis were selected a priori based on evidence of significant unilateral or bilateral group differences in the separate cohort of older PT and FT children and adolescents (Travis et al., 2015a). Fig. 1 shows the analyzed tracts: left (L) and right (R) UF, FMajor, ATR, IFOF and ILF.
Fig. 1.
Tractography of cerebral white matter tracts. Tracts are visualized in a single representative preterm participant. Four left intrahemispheric cerebral tracts are displayed on a mid-sagittal T1 image (A): uncinate fasciculus (UF) = yellow; anterior thalamic radiation (ATR) = purple; inferior fronto-occipital fasciculus (IFOF) = red; inferior longitudinal fasciculus (ILF) = orange. One interhemispheric cerebral tract is displayed on an axial T1 image (B): forceps major (FMajor) = brown. Right hemisphere tract renderings not shown. Dashed lines represent the location of the regions of interest (ROIs) used to isolate each cerebral tract; ROI-1, white, ROI-2, black. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.3. Statistical analyses
2.3.1. Characteristics of PT and FT groups
All statistical analyses were performed using SPSS (version 23.0, IBM Corporation, 2014). Chi-square tests and two-tailed t-tests for independent samples were computed to examine differences between the PT and FT groups on the basis of demographic variables and IQ. Group differences were considered to be significant at p < 0.05.
2.3.2. Primary analyses of fractional anisotropy
Group comparisons were restricted to the same half-tract where significant differences were previously documented in older PT and FT children (Travis et al., 2015a), to reduce the number of comparisons and increase sensitivity. Specifically, we analyzed the medial segment of the UF (nodes 16–30), the left and right segments of the FMajor, the posterior segment of the ATR (nodes 1–15) and the anterior and posterior segments of the IFOF and ILF.
We assessed group differences in FA in these tract segments by calculating a one-tailed t-test for each node, such that the direction of the test was based on prior findings in the older age group. For the IFOF-L, lacking a directional hypothesis, we applied two-tailed t-tests. To control for 15 comparisons performed within each tract segment, we employed a nonparametric permutation-based method (Nichols and Holmes, 2002). This procedure generated a family-wise error corrected cluster size and a family-wise error corrected critical t-value for a single node for each of the candidate tracts. For Protocol 1, the minimum family-wise error corrected cluster size was 3 nodes (range 3–6 nodes) and the minimum family-wise error corrected critical t-value t = 2.36 (range 2.36–2.73). For Protocol 2, the minimum family-wise error corrected cluster size was 3 nodes (range 3–5 nodes) and the minimum family-wise error corrected critical t-value t = 2.26 (range 2.36–2.82). Tract segments were considered significant if differences in FA occurred in (1) a sufficient number of adjacent nodes that met the criteria for a family-wise error corrected cluster size, or (2) nodes in which the effect size was greater than family-wise error corrected critical t-value. Trends for significant group differences were defined as being a minimum of 3 adjacent nodes along FA tract profiles at p < 0.05, uncorrected (Travis et al., 2015a, Travis et al., 2015b).
2.3.3. Secondary analyses: AD, RD, SES and IQ
To investigate the contributions of AD and RD to group differences in FA, we conducted a one-way multivariate analysis of variance (MANOVA) (Travis et al., 2015a). We restricted this analysis to regions that showed group differences after correction in Protocol 1 because it was similar to the acquisition protocol used by Travis et al. (Travis et al., 2015a) For the MANOVA, group (PT versus FT) served as the between-subjects variable and mean AD and mean RD within the significant cluster served as the dependent variables. We chose to enter AD and RD into the same model to reduce the number of comparisons and increase power for detecting group differences. To identify whether significant group effects identified in the MANOVA were the result of group differences in AD, RD or both, we then performed post hoc univariate ANOVAs. We controlled the false discovery rate (FDR) across 10 post hoc comparisons, setting the threshold for corrected significance at p < 0.05 (Benjamini and Hochberg, 1995).
Next, because our sample showed significant group differences in SES and intelligence scores, we assessed whether group differences in FA remained significant after co-varying for SES and IQ. To reduce the number of comparisons, this analysis was again performed only for the 5 tracts found to demonstrate significant group differences after correction in Protocol 1. In this analysis, we calculated a mixed analysis of co-variance (mixed ANCOVA) in which group (PT vs. FT) served as the between subjects factor, mean FA from significant clusters from the 5 tracts mentioned above served as the within subjects factors, and SES and IQ scores served as covariates.
3. Results
3.1. Characteristics of PT and FT groups
The current sample included 20 PT children (11 males, mean age = 6 years 2 months) and 38 FT children (14 males, mean age = 6 years 2 months) (Table SI). Detailed medical information was available for 17 of the 20 PT participants. Medical complications at birth in the PT group were: four with abnormal findings on head ultrasounds or MRIs (3 with grade II-III intraventricular hemorrhage (IVH) and 1 with small periventricular lesions), eleven with mildly abnormal findings (grade 1 hemorrhage); fourteen had respiratory distress syndrome, five developed bronchopulmonary dysplasia (BPD) or chronic lung disease; thirteen had retinopathy of prematurity (ROP) or immature retinae; eleven had patent ductus arteriosus (PDA); ten had hyperbilirubinemia; two were small for gestational age (≤ 3rd percentile birth weight for gestational age). In the present study, a neuroradiologist assessed T1-weighted images collected at age 6 years for 5 features associated with white matter injury: white matter signal abnormality, periventricular (PV) white matter volume loss, cystic abnormalities, ventricular dilation and thinning of the corpus callosum (Travis et al., 2015a, Inder et al., 2003). Excessive punctate lesions were considered mild PV white matter volume loss. We categorized individuals as normal if they had less than or equal to one abnormal feature and abnormal if they had two or more abnormal features. Of the 20 PT subjects, 3 PT participants had abnormal T1-weighted scans at age 6 years, which included the participant with CP.
By design, children in the PT group had significantly decreased GA and birth weight than the FT sample. Children in the PT group did not differ significantly from those in the FT group in age, sex, ethnicity and handedness. SES was significantly higher in the FT than in the PT group. However, on average, both FT and PT participants were from high socio-economic backgrounds. Similar to many other studies, PT children had general intelligence scores within the normal range but had significantly lower IQ scores compared to FT children (Bhutta et al., 2002). Results of these analyses are presented in Table SI. We performed post hoc analyses controlling for SES and IQ to confirm that observed group differences in FA could not be explained by group differences in these measures. We observed positive correlations between gestational age at birth and birth weight in both the combined group (r = 0.96, p < 0.001) and in the preterm sample alone (r = 0.75, p < 0.001). Because of the co-linearity between gestational age and birth weight, we did not to control for individual variations in birth weight in the group comparison, so as not to abolish any effects of prematurity.
3.2. Primary analyses of fractional anisotropy
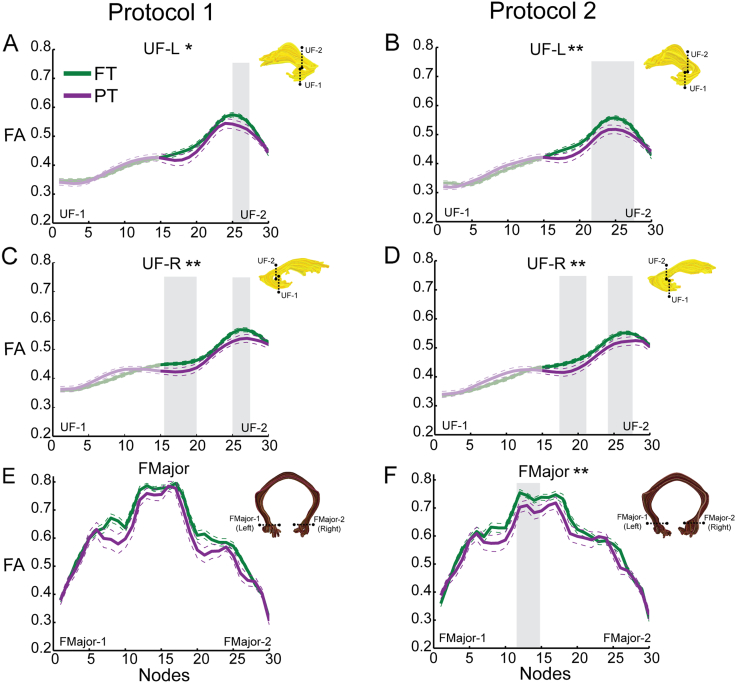
Consistent with our hypotheses, FA was decreased in PT compared to FT children in the medial segment of the UF-L and UF-R, and in the left segment of the FMajor (Table 1; Fig. 2A–D, F). The findings in UF-L and UF-R generalized across Protocols 1 and 2. Group differences within the left segment of the FMajor were found in Protocol 2 only. Group differences did not rise to the level of statistical significance in the right segment of the FMajor nor in the anterior segment of the IFOF-R (Figs. 2E–F; 3E–F).
Table 1.
Locations along the white matter tracts in which preterm and full term groups showed significant group differences in fractional anisotropy in two different protocols.
| 30 diffusion directions |
96 diffusion directions |
|||||
|---|---|---|---|---|---|---|
| Half tract | Significant cluster location | Preterm mean FA of cluster (95% CI) | Full term mean FA of cluster (95% CI) | Significant cluster location | Preterm mean FA of cluster (95% CI) | Full term mean FA of cluster (95% CI) |
| PT mean FA < FT mean FA | ||||||
| UF-L | ||||||
| Medial | 25–27c | 0.53 (0.50–0.56) | 0.57 (0.55–0.58) | 22–27b | 0.51 (0.47–0.54) | 0.54 (0.53–0.56) |
| UF-R | ||||||
| Medial | 16–20a | 0.43 (0.40–0.45) | 0.45 (0.44–0.47) | 17–21a | 0.42 (0.40–0.45) | 0.45 (0.44–0.47) |
| 25–27a | 0.53 (0.51–0.56) | 0.57 (0.55–0.58) | 24–27a | 0.51 (0.49–0.54) | 0.54 (0.53–0.56) | |
| F-Major | ||||||
| Left | – | – | – | 12–15b | 0.70 (0.66–0.73) | 0.74 (0.73–0.76) |
| PT mean FA > FT mean FA | ||||||
| ATR-R | ||||||
| Posterior | 11–15b | 0.56 (0.55–0.58) | 0.54 (0.52–0.55) | – | – | – |
| IFOF-L | ||||||
| Posterior | 10–14b | 0.52 (0.50–0.54) | 0.49 (0.47–0.50) | 11–14b | 0.48 (0.45–0.50) | 0.44 (0.42–0.45) |
| IFOF-R | ||||||
| Posterior | 10–14b | 0.54 (0.52–0.57) | 0.50 (0.49–0.51) | 10–15b | 0.50 (0.47–0.53) | 0.46 (0.44–0.47) |
| ILF-L | ||||||
| Posterior | 6–8c | 0.53 (0.51–0.55) | 0.50 (0.48–0.52) | – | – | – |
| ILF-R | ||||||
| Posterior | 9–14b | 0.51 (0.49–0.53) | 0.48 (0.47–0.49) | – | – | – |
Significant for peak value, corrected, with at least two surrounding nodes.
Significant for cluster size, corrected.
Trend for significance, cluster of at least 3 nodes, uncorrected.
Fig. 2.
Tract profiles in the preterm and full term groups. Mean FA profiles are depicted for each of the cerebral tracts in Fig. 1, for the full term group (solid green line) and the preterm group (solid purple line), from two different dMRI acquisition protocols. Left column (A, C, E) presents data from Protocol 1 (30 directions, b = 1000 s/mm2) and right column (B, D, F) presents data from Protocol 2 (96 directions, b = 2500 s/mm2). A–B: UF-L, nodes 16–30; C–D: UF-R, nodes 16–30; E-F: FMajor, nodes 1–30. For all tracts, node 0 corresponds to ROI-1 and node 30 corresponds to ROI-2. Dashed lines indicate ± 1 standard error of the mean. Shaded gray background indicates nodes where mean FA of the PT group is significantly decreased compared to the FT group. Tracts demonstrating group differences that were significant (p < 0.05) after correcting for multiple comparisons along the tract are indicated with **. Tracts demonstrating a trend toward significant group differences (p < 0.05, uncorrected) are indicated with a single *. Tract renderings from a single subject are shown adjacent to tract profiles for illustration purposes. UF = uncinate fasciculus; FMajor = forceps major; PT = preterm; FT = full term; FA = fractional anisotropy; L = left; R = right. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
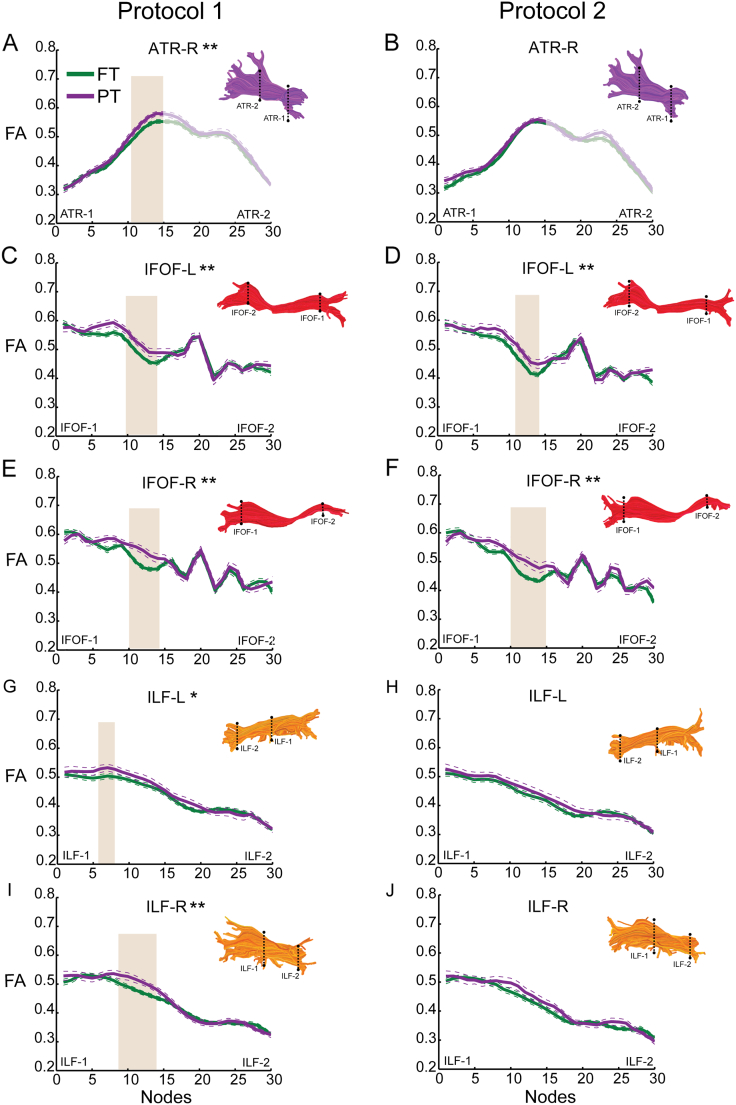
Fig. 3.
Tract profiles in the preterm and full term groups. Mean FA profiles are depicted for each of the cerebral tracts in Fig. 1, for the full term group (solid green line) and the preterm group (solid purple line), from two different dMRI acquisition protocols. Left column (A, C, E, G, I) presents data from Protocol 1 (30 directions, b = 1000 s/mm2) and right column (B, D, F, H, J) presents data from Protocol 2 (96 directions, b = 2500 s/mm2). A–B: ATR-R, nodes 1–15; C–D: IFOF-L, nodes 1–30; E–F: IFOF-R, nodes 1–30; G-H: ILF-L, nodes 1–30; I–J: ILF-R, nodes 1–30. For all tracts, node 0 corresponds to ROI-1 and node 30 corresponds to ROI-2. Dashed lines indicate ± 1 standard error of the mean. Shaded brown background indicates nodes where mean FA of the PT group is significantly increased compared to the FT group. Tracts demonstrating group differences that were significant (p < 0.05) after correcting for multiple comparisons along the tract are indicated with **. Tracts demonstrating a trend toward significant group differences (p < 0.05, uncorrected) are indicated with a single *. Tract renderings from a single subject are shown for each tract for illustration purposes. ATR = anterior thalamic radiation; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; PT = preterm; FT = full term; FA = fractional anisotropy; L = left; R = right. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Consistent with our hypotheses, FA was increased in the PT compared to the FT group in the posterior segment of the ATR-R, IFOF-R, ILF-L and ILF-R (Table 1; Fig. 3A, E–F, G, I). This pattern of findings was replicated in Protocol 2, but group differences only rose to the level of statistical significance in the posterior segment of the IFOF-R (Table 1; Fig. 3F). We also detected increased FA in PT compared to FT children in the posterior segment of the IFOF-L (Table 1; Fig. 3C, D). This pattern was significant in both Protocols 1 and 2. Group differences did not rise to the level of statistical significance in the posterior segment of the ATR-L nor in the anterior segments of the IFOF-R, ILF-L and ILF-R (Fig. 3B, E–J).
Given evidence for abnormal white matter in three PT participants, we performed post hoc analyses to confirm that the results of our primary analysis were not driven by the three preterm participants with abnormal white matter (Supplementary material; Table SII). Specifically, FA was decreased in PT compared to FT children in the medial segment of the UF-L and UF-R, and in the left segment of the FMajor; FA was increased in PT compared to FT children in the posterior segment of the ATR-R, IFOF-L, IFOF-R, ILF-L and ILF-R. Since group differences did not appear to be driven by these three participants with abnormal white matter, all subsequent analyses included these participants.
3.3. Secondary analyses: AD, RD, SES and IQ
In tract segments in which we found a significant group difference in FA, MANOVAs revealed significant multivariate effects of Group (PT vs. FT) in the UF-L, (F = 3.97, p = 0.03), ATR-R (F = 3.56, p = 0.04), IFOF-L (F = 7.02, p = 0.002), IFOF-R (F = 7.75, p = 0.001) and ILF-R (F = 5.12, p = 0.009), but not in the UF-R. The subsequent post-hoc univariate ANOVAs are presented in Table SIII. In the UF-L medial segment, the PT group showed significantly decreased AD compared to the FT group. In tract segments in which FA was significantly increased in the PT group, the PT group had significantly decreased RD compared to the FT group: ATR-R, IFOF-L, IFOF-R and ILF-R. Both AD and RD contributed to increased FA in IFOF-R.
Post hoc analyses confirmed that group differences in FA observed for the UF-R, ATR-R, IFOF-L, IFOF-R and ILF-R were unlikely to be explained by group differences in either SES or IQ. Specifically, the mixed ANCOVA (Group x Tract) analysis revealed a significant main effect of Group (PT versus FT) after controlling for SES and IQ (F = 5.78, p < 0.001).
4. Discussion
Using two dMRI acquisition protocols, we compared tract FA profiles of four bilateral cerebral white matter pathways and one callosal pathway in a sample PT and FT children at age 6 years. Consistent with previous results in PT and FT children at age 9–17 years, segments of the UF-L and UF-R and the left segment of the FMajor demonstrated decreased FA in PT compared to FT children. Segments of the ATR-R, IFOF-R, ILF-L and ILF-R showed patterns for increased FA in PT compared to FT children. Tract FA was also significantly increased in PT compared to FT children at age 6 years in the posterior segment of the IFOF-L. Though group differences in tract FA were not significant in segments of the remaining tracts, there were no segments in which the observed pattern was the inverse of the expected pattern. Post hoc analyses of AD and RD demonstrated that decreased FA in the PT (compared to FT) was driven by significantly decreased AD, while increased FA in the PT (compared to FT) was driven by significantly decreased RD. Group differences in FA were not significantly influenced by group differences in SES or IQ, or by the three participants with abnormal white matter at age 6 years. Thus, the findings in the PT and FT children at age 6 years were very similar to the patterns of differences found in a sample of older children and adolescents whose white matter was analyzed using similar methods, (Travis et al., 2015a) despite differences in the age of participants, dMRI acquisition protocols and MRI scanner. These findings suggest that the complex pattern of PT-FT birth group differences in white matter microstructure is likely an early developing consequence of premature birth.
The patterns of increased and decreased FA seen in the current study are complex. Regions of decreased FA in the PT group could be due to decreased axonal myelination or to less densely packed axons in PT children. Studies performed in non-human animals have demonstrated that between 23 and 32 weeks gestational age, pre-oligodendrocytes are acutely vulnerable to complications of preterm birth, including hypoxia-ischemia, infection and inflammation (Back et al., 2007). Perturbations in the maturation of this cell line may lead to alterations in myelination and contribute to white matter abnormalities observed in preterm children (Volpe et al., 2011, Back et al., 2007). Less dense axonal packing may relate to underdeveloped axons or to a reduced number of axons within the fiber number (Jeurissen et al., 2013, Assaf and Pasternak, 2008, Huppi et al., 2001, Basser and Pierpaoli, 1996). Regions of increased tract FA in the PT group might relate to increased myelination, a compensatory late effect of early injury. More likely, increased FA may be due to a reduced number of crossing fibers or thinner axons in PT children (Groeschel et al., 2014, Jeurissen et al., 2013, Assaf and Pasternak, 2008). Increased FA in PT children may not necessarily indicate increased maturity or greater white matter integrity.
Using an along-tract analytic approach, the present study was able to identify localized PT-FT group differences in FA within specific regions of the UF, IFOF and ILF, bilaterally, and within specific regions of the FMajor and ATR-R. Such differences may have otherwise been obscured had diffusivity measures been averaged across the entire tract trajectory. In the present sample, differences in FA between PT and FT groups were not significant in segments of the FMajor, right IFOF and bilateral ILF in which differences were previously noted (Travis et al., 2015a). The lack of group differences may be due to a smaller group of PT children in this study compared to the older cohort. Alternatively, it may be due to developmental stage: group differences in these tracts may emerge at older ages. Differences in findings between the two dMRI acquisition protocols are likely due to the higher b-value in Protocol 2, which is associated with reduced signal-to-noise ratio (SNR) that may have hindered the ability to detect group differences (Chung et al., 2013). Indeed, there is evidence to suggest that HARDI scans such as those collected in the current study are more likely to reveal group differences if analyzed using constrained spherical deconvolution or other high-order models for diffusion (Reijmer et al., 2012). The present study did not apply such approach in order to directly compare 30 direction to 96 direction scans with the same analytic methods.
Associations between persistent PT-FT differences in WM pathways and neurodevelopmental outcomes have been observed in children born PT. Interestingly, a previous study found that regions showing PT-FT white matter microstructural differences were not in the same tract regions in which associations were found with behavioral outcomes (Travis et al., 2016). Future studies should continue to investigate the functional role of these pathways in relation to behavioral abilities in children born PT. For example, ongoing studies will examine the role of the UF, FMajor, ILF and IFOF in learning to read, as these pathways have been implicated in both language- and reading-related processes (Wandell and Yeatman, 2013, Vandermosten et al., 2012, Ben-Shachar et al., 2007, Catani et al., 2005). Such studies may also help to elucidate whether group differences in the anterior thalamic radiations observed here and in previous tractography studies of preterm neonates contribute to neurodevelopmental outcomes in both full term and preterm children (Liu et al., 2012, Constable et al., 2008). To improve understanding of tissue microstructure driving these differences, studies should include additional dMRI analytic methods, such as constrained spherical deconvolution, to improve resolution of tracking in areas of crossing fibers, and quantitative MRI, to assess myelin content and axonal diameters in areas of both increased and decreased FA (Travis et al., 2015b, Reijmer et al., 2012).
Limitations of this study include a modest sample size. A larger sample size might improve the ability to detect group differences in additional white matter tracts. These children were evaluated on a single occasion at age 6 years. A longitudinal design is required to determine whether differences observed at age 6 years are a consequence of injury during the neonatal period and whether some differences resolve or persist in association with specific clinical or environmental factors.
5. Conclusion
In conclusion, despite patterns of decreased FA in PT compared to FT neonates, by age 6 years and older we see a complex pattern of FA differences. This pattern of increased and decreased FA in PT compared to FT may be due to complex array of neurobiological factors that contribute to FA. Future studies will assess converging evidence from different MRI protocols to identify which neurobiological factors are altered in PT compared to FT children.
The following are the supplementary data related to this article.
Supplementary material
Demographic measures: preterm and full term groups.
Locations along the white matter tracts in Protocol 1 in which preterm and full term groups showed significant group differences in fractional anisotropy excluding participants with abnormal white matter.
Mean AD and RD within segments of the white matter tracts in which group differences in FA reached statistical significance.
Acknowledgments
Acknowledgements
Jenna N Adams assisted with data collection. Wilson Chwang, MD, PhD reviewed MRI scans for white matter injury in PT children.
Acknowledgments
Funding
This work was supported by the National Institute of Child Health and Human Development (Grant # R01HD069162 [Feldman], 5K99HD084749 [Travis]) and the Center of Research Excellence in the Cognitive Sciences (I-CORE Program 51/11 [Ben-Shachar]). Funding sources had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Conflicts of interest
None.
References
- Adams E., Chau V., Poskitt K.J., Grunau R.E., Synnes A., Miller S.P. Tractography-based quantitation of corticospinal tract development in premature newborns. J. Pediatr. 2010;156(6):882–888. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M.C. Neurodevelopmental outcomes of preterm infants. Curr. Opin. Neurol. 2008;21(2):123–128. doi: 10.1097/WCO.0b013e3282f88bb4. [DOI] [PubMed] [Google Scholar]
- Anjari M., Srinivasan L., Allsop J.M., Hajnal J.V., Rutherford M.A., Edwards A.D. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. NeuroImage. 2007;35(3):1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Arzoumanian Y., Mirmiran M., Barnes P.D., Woolley K., Ariagno R.L., Moseley M.E. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. Am. J. Neuroradiol. 2003;24(8):1646–1653. [PMC free article] [PubMed] [Google Scholar]
- Assaf Y., Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Aylward G.P. Neurodevelopmental outcomes of infants born prematurely. J. Dev. Behav. Pediatr. 2005;26(6):427–440. doi: 10.1097/00004703-200512000-00008. [DOI] [PubMed] [Google Scholar]
- Back S.A., Riddle A., McClure M.M. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38(2):724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- Ball G., Boardman J.P., Aljabar P., Pandit A., Arichi T., Merchant N. The influence of preterm birth on the developing thalamocortical connectome. Cortex. 2013;49(6):1711–1721. doi: 10.1016/j.cortex.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57(Series B):289–300. [Google Scholar]
- Ben-Shachar M., Dougherty R.F., Wandell B.A. White matter pathways in reading. Curr. Opin. Neurobiol. 2007;17(2):258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Berman J.I., Mukherjee P., Partridge S.C., Miller S.P., Ferriero D.M., Barkovich A.J. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. NeuroImage. 2005;27(4):862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Bhutta A.T., Cleves M.A., Casey P.H., Cradock M.M., Anand K.J.S. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chung A.W., Thomas D.L., Ordidge R.J., Clark C.A. Diffusion tensor parameters and principal eigenvector coherence: relation to b-value intervals and field strength. Magn. Reson. Imaging. 2013;31(5):742–747. doi: 10.1016/j.mri.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Constable R.T., Ment L.R., Vohr B.R., Kesler S.R., Fulbright R.K., Lacadie C. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- de Kieviet J.F., Pouwels P.J., Lafeber H.N., Vermeulen R.J., van Elburg R.M., Oosterlaan J. A crucial role of altered fractional anisotropy in motor problems of very preterm children. Eur. J. Paediatr. Neurol. 2014;18(2):126–133. doi: 10.1016/j.ejpn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- de la Monte S.M., Hsu F.I., Hedley-Whyte E.T., Kupsky W. Morphometric analysis of the human infant brain: effects of Intraventricular hemorrhage and periventricular leukomalacia. J. Child Neurol. 1990;5(2):101–110. doi: 10.1177/088307389000500206. [DOI] [PubMed] [Google Scholar]
- Feldman H.M., Yeatman J.D., Lee E.S., Barde L.H.F., Gaman-Bean S. Diffusion tensor imaging: a review for pediatric researchers and clinicians. J. Dev. Behav. Pediatr. 2010;31(4):346–356. doi: 10.1097/DBP.0b013e3181dcaa8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano D., Andersen S.K., Partridge J.C., Bonifacio S.L., Xu D., Glidden D.V. Diminished white matter injury over time in a cohort of premature newborns. J. Pediatr. 2015;166(1):39–43. doi: 10.1016/j.jpeds.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeschel S., Tournier J.D., Northam G.B., Baldeweg T., Wyatt J., Vollmer B. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. NeuroImage. 2014;87(15):209–219. doi: 10.1016/j.neuroimage.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Gupta P., Sodhi K.S., Saxena A.K., Khandelwal N., Singhi P. Neonatal cranial sonography: a concise review for clinicians. J. Pediatr. Neurosci. 2016;11(1):7–13. doi: 10.4103/1817-1745.181261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T., Yamada K., Morimoto M., Morioka S., Tozawa T., Isoda K. Development of corpus callosum in preterm infants is affected by the prematurity: in vivo assessment of diffusion tensor imaging at term-equivalent age. Pediatr. Res. 2011;69(3):249–254. doi: 10.1203/PDR.0b013e3182084e54. [DOI] [PubMed] [Google Scholar]
- Hintz S.R., Barnes P.D., Bulas D., Slovis T.L., Finer N.N., Wrage L.A. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–e42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi P.S., Murphy B., Maier S.E., Zientara G.P., Inder T.E., Barnes P.D. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107(3):455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Inder T., Huppi P.S., Zientara G.P., Maier S.E., Jolesz F.A., di Salvo D. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J. Pediatr. 1999;134(5):631–634. doi: 10.1016/s0022-3476(99)70251-9. [DOI] [PubMed] [Google Scholar]
- Inder T.E., Huppi P.S., Warfield S., Kikinis R., Zientara G.P., Barnes P.D. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann. Neurol. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Inder T.E., Wells S.J., Mogridge N.B., Spencer C., Volpe J.J. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J. Pediatr. 2003;143(2):171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J.D., Jones D.K., Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 2013;34(11):2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S., Powell S., He L., Pierson C.R., Parikh N.A. Reliability and repeatability of quantitative tractography methods for mapping structural white matter connectivity in preterm and term infants at term-equivalent age. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0085807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Balériaux D., Kavec M., Metens T., Absil J., Denolin V. Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. NeuroImage. 2010;51(2):783–788. doi: 10.1016/j.neuroimage.2010.02.066. [DOI] [PubMed] [Google Scholar]
- Liu Y., Aeby A., Baleriaux D., David P., Absil J., De Maertelaer V. White matter abnormalities are related to microstructural changes in preterm neonates at term-equivalent age: a diffusion tensor imaging and probabilistic tractography study. AJNR Am. J. Neuroradiol. 2012;33(5):839–845. doi: 10.3174/ajnr.A2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A.M., Neil J.J., Inder T.E. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced MRI. Semin. Perinatol. 2010;34(1):57–66. doi: 10.1053/j.semperi.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannek K., Scheck S.M., Colditz P.B., Boyd R.N., Rose S.E. Magnetic resonance diffusion tractography of the preterm infant brain: a systematic review. Dev. Med. Child Neurol. 2014;56(2):113–124. doi: 10.1111/dmcn.12250. [DOI] [PubMed] [Google Scholar]
- Reijmer Y.D., Leemans A., Heringa S.M., Wielaard I., Jeurissen B., Koek H.L. Improved sensitivity to cerebral white matter abnormalities in Alzheimer's disease with spherical deconvolution based tractography. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0044074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.K., Inder T., Faggian N., Johnston L., Warfield S.K., Anderson P.J. Characterization of the corpus callosum in very preterm and full term infants utilizing MRI. NeuroImage. 2011;55:479–490. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Adams J.N., Ben-Shachar M., Feldman H.M. Decreased and increased anisotropy along major cerebral white matter tracts in preterm children and adolescents. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0142860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Golden N.H., Feldman H.M., Solomon M., Nguyen J., Mezer A. Abnormal white matter properties in adolescent girls with anorexia nervosa. NeuroImage Clin. 2015;9:648–659. doi: 10.1016/j.nicl.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Ben-Shachar M., Myall N.J., Feldman H.M. Variations in the neurobiology of reading in children and adolescents born full term and preterm. NeuroImage Clin. 2016;11:555–565. doi: 10.1016/j.nicl.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Volpe J.J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J., Kinney H.C., Jensen F.E., Rosenberg P.A. Reprint of “the developing oligodendrocyte: key cellular target in brain injury in the premature infant”. Int. J. Dev. Neurosci. 2011;29(6):565–582. doi: 10.1016/j.ijdevneu.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Wandell B.A., Yeatman J.D. Biological development of reading circuits. Curr. Opin. Neurobiol. 2013;23(2):261–268. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Demographic measures: preterm and full term groups.
Locations along the white matter tracts in Protocol 1 in which preterm and full term groups showed significant group differences in fractional anisotropy excluding participants with abnormal white matter.
Mean AD and RD within segments of the white matter tracts in which group differences in FA reached statistical significance.