Fig. 4.
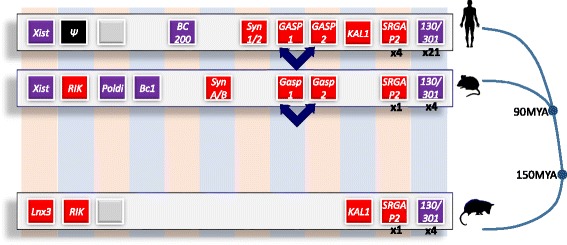
Lineage-specific genes arise through gene birth, death and duplication and exaption of transposable elements. Examples of lineage-specific protein-coding genes (shown in red) or non-coding genes (shown in purple) present in one or more of the human, mouse and opossum (Monodelphis domestica) genomes. Orthologous genes are indicated across vertical columns. Where orthologous sequence is absent, due to a lineage-specific insertion or deletion, no boxes are shown. The eutherian Xist noncoding RNA gene arose, in part, from disruption of an ancestral Lnx3 protein-coding gene [84]. The 2310003L06Rik gene (‘RIK’) is disrupted in human (Fig. 1). The Poldi ncRNA gene arose de novo, within the last ~3.5 million years, in the mouse lineage within untranscribed sequence [85]. Grey boxes indicate non-genic sequence in human and in opossum that is orthologous to the mouse Poldi locus but that has no conservation of transcription. Rodent BC1 and primate BC200 noncoding genes arose independently from separate retrotransposition events yet bind the same protein, FMRP [86]. Similarly, syncytins 1 and 2 arose from endogenous retroviral element insertions in the primate lineage, and separately syncytins A and B arose from such insertions in the rodent lineage [87]. Dark blue double-headed arrows indicate lineage-specific episodes of gene conversion between the two 5′ UTRs of GASP1 and GASP2, genes that are placental mammal-specific [88]. The KAL1 (anosmin-1) gene is entirely absent, and inferred to have been deleted, from the mouse genome [76]. Three duplications of human-specific SRGAP2A/B/C genes occurred within the last approximately 3.4 million years [89]. Four members of the microRNA 130/301 family are present in both the opossum and mouse genome, but 21 members are found in the human genome [36, 90]. MYA million years ago
