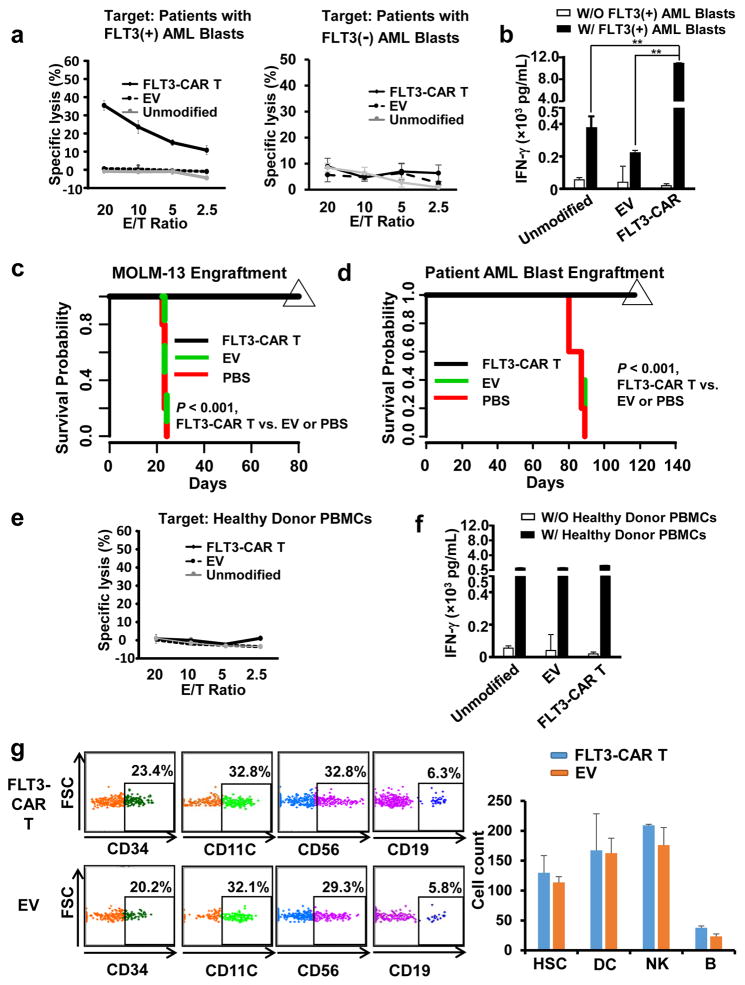
Figure 2. FLT3-CAR T cells show enhanced cytotoxicity and cytokine production against primary AML patient blasts in vitro and against a FLT3(+) AML cell line and patient blasts in vivo, while are not toxic to hematopoiesis.
(a) Cytotoxicity of FLT3-CAR T cells against PBMCs of a representative AML patient containing ~90% FLT3(+) AML blasts, or a representative AML patient containing a comparable percentage of FLT3(−) AML blasts. Similar data of four more patients with ~90% FLT3(+) AML blasts are shown in Supplementary Figure 5. (b) ELISA analysis of IFN-γ secretion by FLT3-CAR T cells against PBMCs of AML patients containing ~90% FLT3(+) AML blasts. Shown here is one patient, representative of all five patients with FLT3 (+) AML blasts. (c) Survival of MOLM-13-bearing mice treated with primary FLT3-CAR T cells, empty vector-transduced T cells, or PBS via tail vein injections. Mice treated with weekly injections of 5 × 106 effector cells for 3 weeks showed 100% survival at 80 days, compared to the two control-treated groups that demonstrated 100% mortality by day 25, as determined by Kaplan-Meier survival curves (n = 5 for each group). (d) 5 × 106 patient PBMC containing ~90% FLT3(+) AML blasts were injected into NSG mice. On day 66 following tumor engraftment, mice were infused with 5 × 106 effect cells weekly for three weeks. Mice treated with weekly injections of 5 × 106 effector cells for three weeks show 100% continued survival at 120 days, compared to the two control-treated groups that demonstrated 100% mortality by day 90, as determined by Kaplan-Meier survival curves (n = 5 for each group). Data presented are representative of three AML patients with similar data. (e). The unmodified T cells, empty-vector transduced T cells, or FLT3-CAR T cells were co-cultured with PBMCs of normal donors for 4 hr, followed by standard 51Cr release assays. Identical effector cells were co-cultured with PBMCs of normal donors for 24 hr after which IFN-γ was measured by ELISA assays. (g). 1 × 106 FLT3-CAR T cells or empty vector-transduced T cells together with 2.5 ×105 human CD34(+) HSCs were simultaneously i.v. injected into NSGS mice that express human IL3, GM-CSF, and SCF. One month and three months later, mice were sacrificed to quantify human CD34(+) HSC and their differentiation as measured by mature lymphocytes and myeloid cells in bone marrow (BM). CD3, CD19, CD56, CD16, and CD14 were used to define lineage cells (Lin). Data shown are for one month engraftment, which are similar to three months’, demonstrating no difference between mice infused with 1 × 106 FLT3-CAR T cells or empty vector-transduced T cells. HSC is defined as CD34(+)Lin(−), DC as CD11c(+)HLDR(+), NK cells as CD56(+)CD3(−)CD19(−), and B cells as CD19(+)CD14(−)CD3(−). n = 4 for EV and n=3 for FLT3-CAR T. “Unmodified” denotes unmodified T cells, “EV” denotes empty vector-transduced T cells, and “FLT3-CAR T” denotes FLT3-CAR-transduced T cells (a–g). ** denotes P < 0.01. Error bars, standard deviation.